The most deadly element of any disease is metastasis, the spread of malignant growth cells all through the body. A new examination by Penn State uncovers interestingly the mechanics behind how bosom malignant growth cells might attack sound tissues. The revelation, showing that an engine protein called dynein powers the development of malignant growth cells in delicate tissue models, offers new clinical focuses against metastasis and can possibly, in a general sense, change how disease is dealt with.
“This disclosure denotes a change in perspective in numerous ways,” said Erdem Tabdanov, partner teacher of pharmacology at Penn State and a lead co-researcher on the review, as of late distributed in the journal Progressed Science. “As of recently, dynein has never been trapped occupied with giving the mechanical power to disease cell motility, which is their capacity to move themselves. Presently, we can see that, assuming you target dynein, you could successfully stop the motility of those cells and, thusly, stop metastatic scattering.”
The undertaking started as a cooperation between Penn State’s Division of Substance Designing and Penn State’s School of Medication, prior to developing into a multi-organization with specialists at the College of Rochester Clinical Center, Georgia Foundation of Innovation, Emory College, and the U.S. Food and Medication Organization.
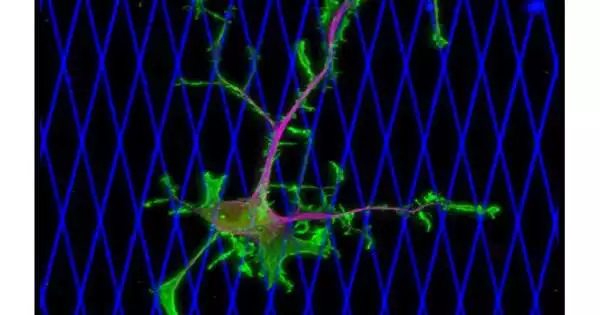
Human bosom disease cells are seen moving inside a 3D model for solid, delicate tissue, planned by Amir Sheikhi of Penn State. The microgels are imperceptible to visual impedance from the cells. The cores of the cells are green. Credit: Erdem Tabdanov/Penn State
The specialists utilized live microscopy to watch the movement of live bosom disease cells in two unique frameworks demonstrated after the human body. The principal framework, a two-layered organization of collagen strands, uncovered how malignant growth cells travel through an extra cell grid that encompasses growths and showed that dynein was critical to the development of disease cells. The subsequent framework was a three-layered model created by a group led by Amir Sheikhi, Dorothy Foehr Huck, and J. Lloyd Huck Early Vocation Seat in Biomaterials and Regenerative Designing and an assistant teacher of compound design and biomedical design at Penn State.
“With chemotherapy, the goal is to kill cancer cells slightly faster than the rest of the body—it’s a race against time. While it is busily eradicating the cancer, chemotherapy does significant damage to the body’s normal, healthy tissues. We could keep the healthy parts of the body healthy if we confined the cancer and halted it in its tracks.”
Erdem Tabdanov, assistant professor of pharmacology at Penn State.
The subsequent framework was intended to imitate delicate tissue by utilizing an organization of tiny hydrogel particles or microgels connected together in growth-like shapes. Like in the two-layered model, the specialists found in the three-layered model that dynein was “key” in the spread or metastasis of disease cells.
“Utilizing these three-layered models that to some extent imitate a growth, that’s what we found; assuming that we block the dynein, the disease cells can’t really move and penetrate strong tissues,” Sheikhi said. “In the two models, we found that dynein is critical for cell movement, which proposes an entirely different technique for disease management. Rather than killing the disease cells with radiation or chemotherapy, we are telling them the best way to die. This is incredible news since you don’t actually need to kill the cells, which is an unforgiving methodology that targets both carcinogenic and solid cells. All things being equal, you simply need to prevent the disease cells from moving.”
Tabdanov made sense of the fact that cell “loss of motion” could end up being a successful therapy methodology for disease compared with chemotherapeutic therapies, in light of the fact that after careful evacuation of the fundamental growth, it could keep the disease from spreading without harming sound tissues and cells.
“The stunt with chemotherapy is to kill the malignant growth cells somewhat quicker than the remainder of the body—it’s a test of skill and endurance,” Tabdanov said. “Chemotherapy does a ton of harm to the body’s ordinary, solid tissues while it is occupied with killing the malignant growth. In the event that we rather contained the disease and left it speechless, we could keep the sound pieces of the body solid.”
The scientists noticed that any potential clinical treatment is still far away, as they presently can’t seem to run human or creature preliminaries. Sheikhi has documented numerous licenses connected with his group’s foundation and plans to utilize the innovation to concentrate on a heap of illnesses, including different tumors.
“We are extremely amped up for this joint effort with the Penn State School of Medicine, and our labs are working intently on different activities,” Sheikhi said. “I figure these stages might one day, at any point, empower customized medication and customized therapy for malignant growth and, ideally, numerous different illnesses.”
Different creators on the paper are Yerbol Tagay of Penn State School of Medication; Sina Kheirabadi and Zaman Ataie of Penn State’s Branch of Synthetic Designing; Rakesh Singh of the College of Rochester Clinical Center; Denis Tsygankov of Georgia Foundation of Innovation and Emory College; and Olivia Sovereign, Ashley Nguyen, Alexander Zhovmer, and Xuefei Mama of the U.S. Food and Medication Organization.
More information: Yerbol Tagay et al. Dynein-Powered Cell Locomotion Guides Metastasis of Breast Cancer, Advanced Science (2023). DOI: 10.1002/advs.202302229