Bone marrow transfers have saved the lives of thousands of blood malignant growth patients, yet join-versus-have sickness, or GVHD, remains a crippling and, surprisingly, hazardous inconvenience. Another review from the Fred Hutchinson Malignant Growth Community focuses on patients’ pre-relocated stomach microbiomes as a key factor in the turn of events and seriousness of GVHD.
In the review, distributed in Resistance, the exploration group utilized creature models of GVHD and complex computational examinations to distinguish microbes that assist with affecting GVHD from others that stifle it. Microbiome information from blood disease patients who got bone marrow transfers recommended that a portion of similar bacterial gatherings may likewise impact GVHD in individuals.
“Many examinations on GVHD and the microbiome center around the microbiome after relocation,” said Motoko Koyama, MD, Ph.D., a staff researcher in Fred Cubby’s Slope Lab who drove the review. “Our work shows that the pre-relocated microbiome could be the focal point of treatments to lessen the seriousness of GVHD.”
“The microbiome after transplant is the focus of many studies on GVHD and the microbiome. Our findings indicate that the pre-transplant microbiota could be the focus of therapy to lower the severity of GVHD.”
Motoko Koyama, MD, Ph.D., a staff scientist in Fred Hutch’s Hill Lab who led the study.
The work assists scientists with better comprehension of how GVHD creates and where to concentrate further translational investigations aimed at forestalling this frequently destructive result. Specifically, the discoveries highlight ways to tweak this pathway that can be tried clinically, said Geoff Slope, MD, who coordinates Hematopoietic Undeveloped cell Transplantation at Fred Cubby.
Unraveling a dangerous transfer intricacy
At the point when blood malignant growths like leukemia strike, white platelets that ought to battle disease have lost themselves and started outgrowing control.
Bone marrow is the wellspring of our insusceptible cells, our oxygen-conveying red platelets, and our coagulation-shaping platelets. To treat leukemia, specialists clear out both malignant, insusceptible cells and the bone marrow that can re-seed them, supplanting them with solid, immature microorganisms that will flourish (engraft) and produce a fresh, safe framework.
Giver resistant cells complete two things: They assist with supplanting destructive resistant cells, and they assault waiting leukemia cells in what’s known as the join versus leukemia, or GVL, impact.
Sadly, they now and again cause GVHD by going after solid beneficiary cells. Side effects of this frequently incapacitating condition include regurgitation, looseness of the bowels, jaundice, skin rashes, and an elevated chance of contamination. Seriousness goes from gentle to destructive. Intense GVHD emerges in no less than 100 days after relocation and frequently happens at areas where the body meets—and repulses—the outside world, similar to the stomach and the skin. GVHD is the main cause of non-backslide deaths in bone marrow transplant patients, and around 5,500 patients foster GVHD every year.
Researchers don’t totally comprehend the reason why this is so, yet obviously the organisms that call these regions home contribute to GVHD frequency and seriousness.
Koyama and Slope need to see precisely the way in which microscopic organisms associate with the resistant cells that cause harm. In a past report, Koyama uncovered that, startlingly, the epithelial cells coating the stomach can give resistant cells the assistance they need to prompt GVHD.
That work uncovered the significance of a resistant particle called interleukin 12. The discoveries roused a group led by Fred Pen bone marrow relocation master Stephanie Lee, MD, MPH, who holds the David and Patricia Giuliani/Oliver Press Blessed Seat in Malignant Growth Exploration, to embrace clinical preliminary testing of a GVHD-preventive system pointed toward repressing IL-12.
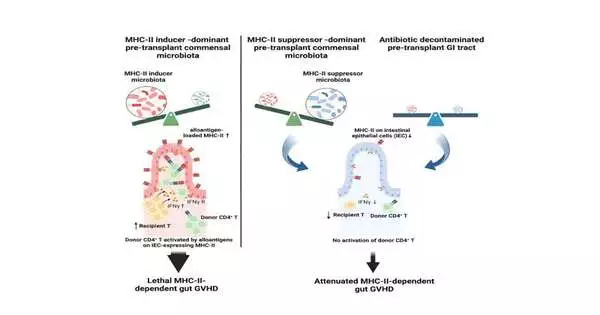
Koyama and the group likewise found that epithelial cells turned on a particle called MHCII, or significant histocompatibility complex class II, that permits them to speak with resistant cells. She saw that mice raised without a microbiome had practically no MHCII in their stomach epithelial cells and, furthermore, didn’t get stomach GVHD, proposing that microorganisms were playing a significant role.
Microorganisms can help or frustrate GVHD.
“We knew that the microbiome was significant in causing GVHD by means of MHCII, but we didn’t know which bacterial species were involved,” Koyama said.
To assist her with uncovering the bacterial players in GVHD, Koyama exploited the way that the stomach microbiome is molded by ecological variables like food and water.
Over the long haul, changing these elements changes the microbiome’s cosmetics. The group overviewed hereditarily indistinguishable lab mice developed by various sellers and found that mice brought up in various areas have different microbiomes and various degrees of MHCII in their stomach epithelium, and that mice with more elevated levels of stomach MHCII get more extreme GVHD.
To limit which bacterial species were engaged in creating intense GVHD through MHCII, the scientists handled the issue from two sides: by adding microscopic organisms and removing them.
In the first place, Koyama and Slope collaborated with microbiome master Sujatha Srinivasan, Ph.D., in Fred Pen’s Fredricks Lab. The colleagues utilized hereditary sequencing to examine the bacterial species present in mice from various sellers. Koyama teamed up with computational scholar Daniel Hippe, Ph.D., in Fred Pen’s Randolph Lab to breakdown the relationships between bacterial gatherings and MHCII. They saw that specific bacterial genera—an ordered gathering more extensive than species—sought to incite MHCII articulation, while others stifled it.
Then, at that point, Koyama put mice with various degrees of stomach MHCII in similar enclosures.
“This moves the microscopic organisms [between the mice], and we can perceive how this changes MHCII articulation,” Koyama said.
After a month, the MHCII-low mice had changed into MHCII-high mice, and Koyama and Hippe saw that MHCII-initiating microscopic organisms had moved into their stomach microbiomes. She was likewise ready to dispense with “inducer” microorganisms and pack down MHCII and GVHD, utilizing specific anti-infection agents preceding transfer.
Tragically, Koyama additionally saw that treating mice with these anti-toxins likewise diminished GVL, the capacity of the giver cells to go after remaining leukemia cells, albeit less seriously than GVHD. In patients, GVL helps hold their malignant growths back from repeating, so treatment systems should balance the advantage of lessening GVHD with the gamble of diminishing GVL and upgrading repeat.
“Another gathering had previously shown that specific microorganisms increment MHCII articulation on stomach epithelial cells,” Koyama said. “We had to be aware: do the silencer microorganisms make a utilitarian difference?”
Working with Cubby associate and microbiome master Neelendu Dey, MD, the group grew two kinds of silencer microscopic organisms. Koyama pretreated two gatherings of MHCII-high mice with anti-microbials to clear out the vast majority of the “inducer” microorganisms.
Fourteen days of everyday dosing with the silencer microscopic organisms sloped down their stomach epithelial MHCII levels, while MHCII levels returned quickly in mice that got anti-toxins but no silencer microbes. Mice that got the silencer microorganisms after anti-toxin treatment likewise endured better after relocation.
Koyama then, at that point, teamed up with another Fred Box microbiome researcher, Kate Markey, Ph.D., MClin, to check whether the human information upheld her discoveries in mice.
They broke down a companion of microbiome information taken from bone marrow transplant patients preceding transfer at Remembrance Sloan Kettering Malignant Growth Place in New York City. While individual species contrasted an extraordinary arrangement among mice and human stomach microbiomes, the scientists saw particular cross-over between GVHD-related bacterial genera in mice and people.
“This proposes that before relocating, people likewise have various microorganisms in their stomach microbiota that can impact GVHD results,” Koyama said.
An open door
While the group’s discoveries feature the force of the microbiome in advancing GVHD, it will probably be some time before specialists will actually want to adjust this perplexing environment to further develop the results, she said.
Yet, by explaining how connections between the microbiome and benefactor and host-safe cells encourage GVHD, the scientists’ work uncovers the vital associations and players that can then become the focal point of additional investigation into helpful targets and new treatment systems.
“There is still a great deal to comprehend about how to control the microbiome, yet our work shows that the pre-relocate microbiome is a significant spot to concentrate future examination,” Koyama said.
More information: Motoko Koyama et al, Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity, Immunity (2023). DOI: 10.1016/j.immuni.2023.06.024