In another review, scientists at the College of Maryland have demystified the cycle by which cells accept their shape—aand everything begins with a protein called actin.
Actin is a critical part of the cytoskeleton that gives structure to cells, similar to how our skeletons support our bodies. Nonetheless, not at all like our skeleton, the actin cytoskeleton is a profoundly flexible construction that can quickly collect and dismantle in light of biochemical and biophysical signals.
It is notable that actin can frame both 3D round shell-like designs that safeguard cells from outer strain and 2D rings that adjust intracellular capabilities. Yet, at whatever point specialists attempted to reproduce these designs outside the cell, they quite often wound up with groups of actin. Nobody knew whyhaas of not long ago.
“Actin rings and spherical shells are found in nearly all cell types across all species. Understanding the mechanism underlying the development of these structures, we believe, will open the door to understanding how cells sense and respond to their surroundings.”
Garegin Papoian, a co-author of the study and a UMD Monroe Martin Professor in the Department of Chemistry
The scientists utilized virtual experiences to show that actin and its accomplice protein, myosin, take part in a back-and-forth, with myosin attempting to trap actin in nearby groups and actin endeavoring to escape. In the event that actin wins, actin fibers get away from myosin’s pulling force and precipitously structure rings and circular shells. On the off chance that myosin wins, the actin network implodes and forms thick bunches.
“Actin rings and circular shells are universal in practically all phone types across species.” “We imagine that understanding the system behind the development of these designs opens the way to how cells sense and answer their current circumstance,” said Garegin Papoian, a co-creator of the review and an UMD Monroe Martin Teacher in the Division of Science and Organic Chemistry and the Organization for Actual Science and Innovation (IPST).
Their discoveries, distributed Oct. 21, 2022, in the electronic diary eLife, could have significant ramifications for human wellbeing. Since actin rings are fundamental to our bodies’ capacity to ward off unfamiliar cells, which could otherwise bring about debilitated invulnerability or immune system issues, the discoveries of this study could help the improvement of future medications.
Actin monomers can be considered railroad vehicles, which connect up to frame a train-like actin fiber. These actin trains travel through the phone in a cycle called treadmilling. Likewise influencing everything are the myosin engines, which pull trains in opposite directions toward one another. Papoian, Qin Ni (Ph.D. ’21, substance designing), and biophysics Ph.D. understudy Haoran Ni accepted that a rivalry between myosin’s pulling force and the pace of treadmilling was responsible for the development of actin rings.
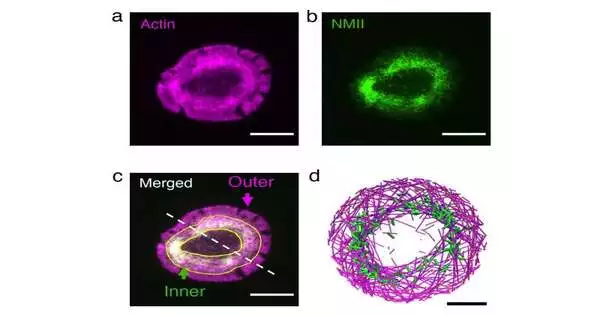
Calibrating these boundaries in living cells is unimaginable, so the specialists turned to a reproduction program called Medyan, created by the Papoian Lab. MEDYAN utilizes material science and science rules to recreate the elements of cytoskeletal proteins. They reproduced an actin and myosin organization (by and large alluded to as “actomyosin”) in a dainty circle and circular shell.
They saw that, assuming the actin trains move gradually, the myosin pulling force causes gridlocks, which are the actomyosin bunches that have been seen in networks of reconstituted external cells. Then again, assuming the actin trains move quickly, they can get away from myosin’s draw. When they arrive at the limit of the circle, myosin’s pulling force makes the actin trains turn, preventing a head-on impact with the plate edge. A replay of these occasions brings about every one of the trains moving in a circle along the border of the plate, which frames the actin ring.
Further investigation offers a thermodynamic hypothesis to make sense of why cells form rings and shells. As indicated by the laws of physical science, frameworks favor the least-energy arrangement. Myosin proteins produce a ton of mechanical energy by bowing actin fibers, which must be delivered in the event that actin can take off and unwind. In living cells, actin’s capacity to move quickly enough to get away from myosin and rush to the edge considers this developed energy to be delivered, taking into consideration the arrangement of rings or shells, which, thermodynamically speaking, is the most minimal energy setup.
“The explanation that the rings were not recently seen outside the phone is on the grounds that actin simply wasn’t moving quickly enough,” Papoian said. “Myosin was winning multiple times out of 10.”
Along with Arpita Upadhyaya, an UMD physical science teacher with a joint arrangement in IPST, and physical science graduate understudy Kaustubh Wagh, organic sciences graduate understudy Aashli Pathni, and biophysics graduate understudy Vishavdeep Vashisht, the group set off on a mission to test this model in residing cells by directing their concentration toward lymphocytes, where rings normally structure.
Immune system microorganisms are the cells in our body that chase down unfamiliar cells. At the point when they perceive a phone as unfamiliar and become initiated, the lymphocyte cytoskeleton quickly rearranges itself to frame an actin ring at the phone’s cell interface. Beginning with cells that had shaped rings, the analysts explored the impact of annoying actin and myosin utilizing high-resolution live-cell imaging.
Lessening the actin train speed brought about disintegration of the ring into little groups, while expanding myosin’s pulling force prompted fast constriction of the ring, in astounding concurrence with related reproductions.
As a development from this review, the group intends to add greater intricacy to the model and incorporate other cytoskeletal parts and organelles.
“We have had the option to catch one basic part of cytoskeletal association,” Papoian said. “Piece by piece, we intend to construct a computational model of a total cell utilizing major standards from material science and science.”
More information: Qin Ni et al, A tug of war between filament treadmilling and myosin induced contractility generates actin ring, eLife (2022). DOI: 10.7554/eLife.82658
Journal information: eLife