An exploration group led by Prof. Chen Chunying from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences (CAS) has as of late examined the development of the nano-protein crown during endocytosis and its unsettling influence on protein homeostasis and cell digestion. Their outcomes were distributed in PNAS.
When nanoparticles enter organic frameworks, biomolecules of natural liquid rapidly tie to the outer layer of the nanoparticles. The nano-protein crown shaped by connecting with protein particles in the blood as a beginning advance tremendously affects the vehicle and destiny of the nanoparticles. What the development of the nano-protein crown means for the acknowledgment, transport, circulation, work and organic impacts of nanoparticles in the tissues and cells of various obstruction frameworks is a “black box” for clinical use of nanomaterials, which confines the conveyance proficiency of nanomedicine, yet in addition genuinely influences viability and security.
“This research elucidates the evolution of nanoparticles from blood to a subcellular microenvironment and determines the peculiarity of the intracellular microenvironment of nano-protein corona, changing cell metabolism.”
Prof. Chen Chunying from the National Center for Nanoscience and Technology (NCNST)
A significant test in this space is the intricacy of the nano-protein crown, which is impacted by the variety of biomolecules in various tissues and organs, as well as physiological and neurotic states. As of now, there is a critical need to comprehend how the protein piece and underlying qualities of the protein crown develop inside organic microenvironments.
To take care of this issue, the specialists have uncovered a unique advancement example of the protein organization of the nano-protein crown during the time spent cell transport through the imaginative use of complex multi-omics (proteomics, metabolomics, and lipidomics), intermolecular associations, and in situ mass spectrometry imaging.
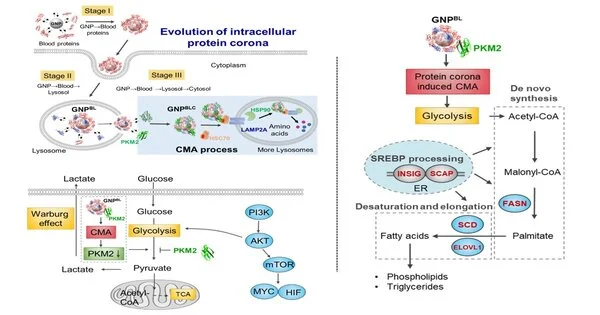
Taking gold nanoparticles as the model, the unique advancement interaction of the protein crown from the blood framework to the intracellular (blood-lysosomal-cytoplasm) was considered. When the nanoparticles were endocytosed into the lysosome from the blood climate, and afterward got away from the lysosome into the cytoplasm, the protein structure on the outer layer of the nanoparticles would change emphatically. Most were replaced by intracellular protein atoms, which held only a portion of the protein crown parts that were framed in the blood environment.
Consequently, intracellular development of the nano-protein crown disturbed intracellular protein homeostasis (proteostasis), yet in addition, set off the enhancement of chaperone proteins (HSC70, HSP90), and pyruvate kinase M2 (PKM2) on the outer layer of the intracellular nano-crown, and animated chaperone-interceded autophagy. It further impacted cell glycolysis, making changes to the cell energy digestion and controlling the cell lipid digestion process.
This study clarifies the transformative example of nanoparticles from blood to a subcellular microenvironment and recognizes the explicitness of the intracellular microenvironment of the nano-protein crown, subsequently reshaping cell digestion. It also provides hypothetical assistance for a comprehensive understanding of the complex organic effects of nanomaterials and nanobiotic interface guidelines.
More information: Rong Cai et al, Dynamic intracellular exchange of nanomaterials’ protein corona perturbs proteostasis and remodels cell metabolism, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2200363119