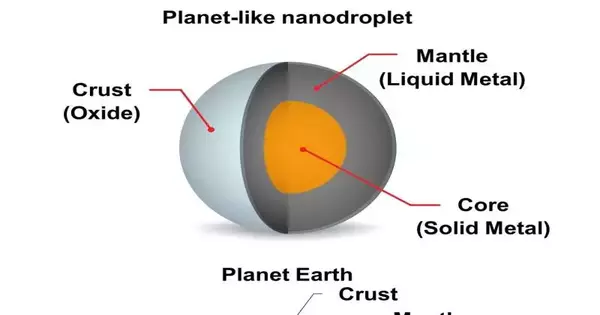
A new method developed at Australia’s RMIT University has resulted in the successful formation of liquid metal nanodroplets with the appearance of planets. Like our own planet Earth, the nanodroplets include an external “outside layer,” a fluid metal “mantle,” and a strong “center.”
The strong intermetallic center is the way to accomplish a more homogenous blend by “securing” a similar measure of solute (i.e., the “target” metals) in each alloyed drop.
The examination group achieved homogeneity through complete disintegration inside the fluid metal medium, made conceivable by high-temperature liquid salt. Their article, “Combination of planet-like fluid metal nanodroplets with promising properties for catalysis,” was distributed in Cutting Edge Practical Materials in July 2023.
Flexible electronics, phase-change materials, catalysts and fuel cells, silver-based antimicrobials, and other applications are just a few of the many areas where this discovery opens up new research opportunities in fundamental liquid-metal chemistry.
“In extreme circumstances, many or even most nanodroplets may be entirely devoid of the solute metal, which ends up being concentrated in only a few particles,”
Dr. Torben Daeneke, also at RMIT.
Liquid metal nanodroplets shake apart
As a novel reaction interface for solvents and catalysts, liquid metals have emerged as a promising new field of chemical research in recent years. These nanodroplets of liquid metal shake apart.
Due to their soft, fluid interior and delocalized metallic bonds, they can also function as a material with high conductivity.
The synthesis of liquid metal nanodroplets has emerged as a significant area of focus in light of the growing number of applications in nano-electronics, sensing, and catalysis that require large surface areas.
When alloying for specific purposes, numerous combinations are possible, such as dissolving copper (the solute) in gallium (the metallic solvent) in liquid form.
The fluid metal nanodroplets are made by mechanical fomentation involving sound waves in a dissolvable liquid like ethanol or water.
However, liquid-metal alloys have a tendency to “de-alloy,” or separate into their individual metals, during this “sonication” process.
This is because previous approaches attempted to dissolve the metals at temperatures close to room temperature. Similarly, as it’s feasible to break up more sugar in warm water than in chilly water, more copper can be broken down in hotter gallium,” says lead creator Caiden Parker, a Ph.D. applicant at RMIT.
Before completely dissolving, some of the solute metal re-forms into larger, solid particles at low temperatures.
The resulting composition has properties that are inconsistent and inhomogeneous, and the composition of each nanodroplet varies significantly. According to corresponding author Dr. Torben Daeneke, who is also at RMIT, “In extreme cases, many or even most nanodroplets may be essentially devoid of the solute metal, which ends up being concentrated in only very few particles.”
This inhomogeneity and the presence of intermetallic intensities present extensive challenges for scientists wishing to figure out the principal components at work in fluid metal science.
High temperatures and salts structure homogenous, planet-like nanodroplets.
In the new review, RMIT scientists settled the issue of dealloying by essentially warming the combination cycle (as high as 400°C) to guarantee the solute metal is totally disintegrated and presenting a painstakingly chosen liquid salt suspension liquid.
The sodium acetic acid derivation was chosen since it stays stable at high temperatures and can be effortlessly taken out subsequently.
The subsequent nanodroplets highlight an intriguing planet-like construction comprising an external (oxide) shell, a fluid (metal) mantle, and a suspended, strong focal center (intermetallic).
“We were promptly struck by the nanodroplets’ similitude to an Earth-like planet, with a strong external shell, a fluid metal mantle, and a strong metal center,” says Caiden.
The new method’s success is dependent on that solid core, which “locks up” the same amount of solute in each alloyed droplet.
Caiden goes on to say, “We were also delighted to see that our new metallic planet-like nanodroplets were everywhere.”
The output yield had significantly increased as a result of the system’s uniform distribution. Transmission Electron Magnifying Lens (TEM) Examination: The center construction is seen in pretty much every bead.
The planet-like nanodroplets can also be used to speed up chemical reactions in a very interesting way thanks to the solid core’s presence.
Electro catalytic ethanol oxidation using the investigated copper-gallium nanodroplets showed promising results that could be utilized in ethanol fuel cells.
Expulsion of the sodium acetic acid derivation is significant before this synergist response, with the salt effortlessly cleaned away in straightforward water showers.
What follows?
High-surface-area nanodroplets can now be used in a wide range of future fields, including electronics and catalytic materials, thanks to this exciting new method.
The actual size of the nanodroplets (i.e., nano as opposed to miniature) will likewise help major investigations of fluid metal science, including investigating the exact idea of bond arrangement inside fluid metals, solvation capacities, crystallization elements, and the overall colloidal science that might happen inside different liquid metal frameworks.
“The planet-like designs resemble minimal small-scale research centers, permitting us to concentrate on how liquid metals act at a nuclear level,” says Torben.
While the review demonstrated the reasonability of the new procedure utilizing a copper-gallium framework, the creators anticipate that further work should affirm that the strategy will find success utilizing different mixes of solute and dissolvable combination frameworks, starting with silver, zinc, or bismuth in fluid gallium, tin, or indium.
According to Caiden, “the ability to adjust the metal mix for certain applications, dependent on the properties of the constituent metals, is a key advantage of liquid-metal systems.”
Copper, for instance, is an excellent electrical conductor. At the point when we join copper with gallium, we save tremendous expense in material utilization, yet in addition, we open the way to adaptable hardware, for example, what you could have seen in science fiction motion pictures.”
Copper might also be used for its thermal properties, and nanodroplets made of copper could be used in systems for dissipating heat.
Nanodroplet catalysis applications in view of the capacity of copper to accelerate responses have previously been tried in the new review, with further development of the dynamic site region notwithstanding material blend reserve funds.
Taking a gander at another metal, silver has recently found applications in view of its antimicrobial properties, and once joined with gallium, it could make a more bioavailable alternative.
As a result, the new technology has a wide range of applications. The system can be utilized by any industry that requires nanomaterials, with application-specific variations in the constituent metals,” claims Torben.
More information: Caiden J. Parker et al, Synthesis of Planet‐Like Liquid Metal Nanodroplets with Promising Properties for Catalysis, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202304248