While agony and dread are altogether different encounters, past investigations have demonstrated the way that they can, in some cases, be firmly connected with each other. For instance, when a lot of animals and people are in dangerous or life-threatening situations, acute fear can prevent them from feeling pain and allow them to fully concentrate on what is going on.
On the other hand, research showed that when people experience elevated degrees of agony, they can make long-term and cooperative trepidation recollections that make them unfortunate of the circumstances that they associate with the aggravation they felt. These memories can, in turn, make them more sensitive to pain or cause them to develop unhelpful ways of acting that try to avoid it.
It’s possible that animals or humans’ fearful anticipation of pain is analogous to the increased intensity with which they perceive pain following extremely painful previous experiences. However, the precise neural foundations of this process are still poorly understood.
“We demonstrate in mice that a painful episode shapes pain experience later in life depending on long-term associative fear memory stored in neuronal engrams in the prefrontal cortex,”
Alina Stegemann, Sheng Liu and their colleagues wrote in their paper.
A recent study by Heidelberg University researchers sought to better comprehend which regions of the mice’s brain store extremely painful experiences and how these stored memories can influence future pain experiences. The prefrontal cortex, which covers the front of the mammalian brain, may be where these memories are stored, according to their findings, which were published in Nature Neuroscience.
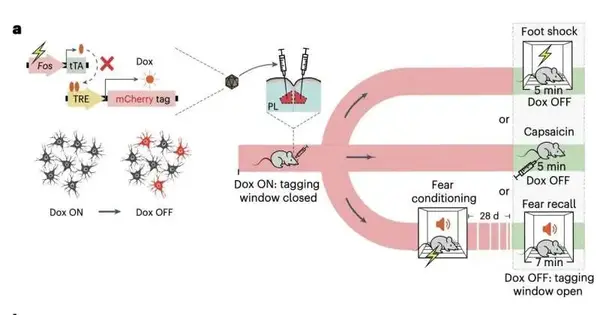
Using optogenetics and neural tagging, the researchers carried out a series of experiments on adult mice. The mice were conditioned to become afraid of getting small electric shocks on their feet again during these experiments. Additionally, in order to ascertain how this would affect the mice’s sensitivity to pain, the team employed optogenetic techniques to either activate or suppress various neural circuits in the brain.
“We show in mice that drawn-out cooperative trepidation memory put away in neuronal engrams in the prefrontal cortex decides if an excruciating episode shapes torment experience sometime down the road,” Alina Stegemann, Sheng Liu, and their partners wrote in their paper.
“Besides, under states of provocative and neuropathic torment, prefrontal trepidation engrams extend to envelop neurons addressing nociception and material sensation, prompting articulated changes in prefrontal availability to fear-applicable cerebrum regions. Conversely, chronically established hyperalgesia and allodynia are reversed when prefrontal fear engrams are silenced.”
This group of researchers has recently done some research that outlines some of the neural mechanisms that could be involved in the long-term persistence of pain caused by the formation of fearful associative memories of past pain. New therapeutic approaches for chronic pain manifestations that can be linked to previous painful experiences could be inspired by their findings. These restorative mediations could, for example, combine mental and social treatment with drugs focusing on brain circuits in the prefrontal cortex.
Stegemann, Liu, and their colleagues wrote in their paper, “These results reveal that a discrete subset of prefrontal cortex neurons can account for the debilitating comorbidity of fear and chronic pain and show that attenuating the fear memory of pain can alleviate chronic pain itself.” An impetus for the development of interventions that target prefrontal circuitry in people who have chronic pain and comorbid fear is provided by our study, which provides causal evidence that overpowering anticipatory fear reduces pathological pain and spurs further research into the subject.
More information: Alina Stegemann et al, Prefrontal engrams of long-term fear memory perpetuate pain perception, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01291-x