Manganese oxides are naturally reactive minerals that are common in both aquatic and terrestrial environments. Through adsorption and oxidation in sewage treatment, they have an impact on the fate of metals (like As3 and Cd2) and organic pollutants (like phenols and diclofenac). Most of the time, it is believed that dissolved Mn(II) is oxidized by abiotic or biotic processes to produce manganese (III or IV) oxides in the environment.
Although the reaction between dissolved Mn(II) and Mn(III/IV) oxides has a high energy barrier, it is thermodynamically advantageous to oxidize aqueous Mn(II) by dissolved oxygen. However, the reaction’s kinetics are slow. As a result, microorganisms are thought to be the primary source of manganese oxides in the environment because they speed up oxidation, which is 4–5 orders of magnitude faster than the rate of abiotic chemical oxidation.
Usually referred to as “manganese-oxidizing bacteria,” these microorganisms have the ability to catalyze the conversion of dissolved Mn(II) ions to undissolved Mn(III/IV) oxides. Direct oxidation, which is the process catalyzed by enzymes on the surface of microorganisms, is one of two ways that bacteria can oxidize Mn(II) ions. Some bacteria can alter their surrounding environmental conditions for Mn(II) oxidation in indirect pathways (e.g., pH and Eh).
Recent research has shown that the Roseobacter clade can oxidize Mn(II) by releasing extracellular reactive oxygen species. Is Roseobacter’s Mn(II) oxidation closely related to the physiological process of bacteria, or do other bacterial clades have different Mn(II) oxidation processes?
These queries will be addressed by Prof. Using coastal surface seawater microorganisms, Feng Zhao from the Chinese Academy of Sciences and his team investigated the microbial manganese oxidation process under visible light. Microorganisms’ conversion of soluble Mn(II) into insoluble Mn(III/IV) oxides and their physiological function were studied in relation to one another. In 2023, the results of this study will be published in Frontiers of Environmental Science and Engineering.
The research team discovered that visible light significantly accelerates the oxidation rate of Mn(II), with an average rate of 64 mol/(Ld). Biological function accounts for 88 percent of the rapid manganese oxidation, which was caused by the combined action of biotic and abiotic factors. The generated manganese oxides were then favorable for Mn(II) oxidation.
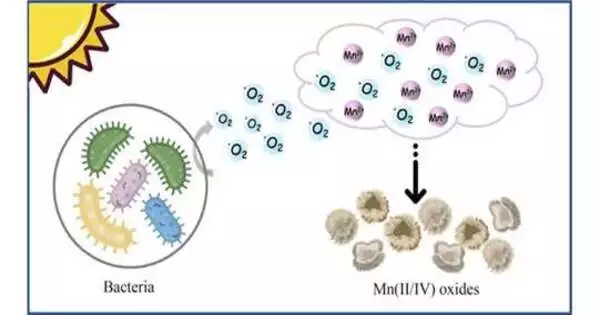
The key element in our study’s rapid manganese oxidation is extracellular superoxide created by microorganisms stimulated by visible light. However, the presence of Mn(II) ions is not necessary for the production of these superoxides; rather, the Mn(II) oxidation process was more like an accidental side reaction that had no impact on the development of microorganisms.
Based on the oxidizing abilities of free radicals, more than 70% of heterotrophic microorganisms in nature are capable of producing superoxide, and all of these bacteria can take part in the geochemical cycle of manganese. A significant natural source of manganese oxide may also come from the superoxide oxidation pathway.
The pathway for bacterial manganese oxidation was identified by this study. The main source of manganese oxides is oxidized Mn(II) ions in the environment, which are produced by heterotrophic bacteria when exposed to visible light. Under light illumination, the biogenerated Mn(III/IV) oxides can also oxidize Mn(II) ions indirectly through abiotic processes.
It is possible that many environmental bacteria that actively or passively produce superoxide also oxidize Mn II in this manner, indicating that the environment frequently engages in the manganese oxidation pathway through superoxide. This study will offer fresh suggestions for combating environmental pollution because of the oxidation and semiconductor properties of manganese oxides.
More information: Fan Yang et al, Visible light induces bacteria to produce superoxide for manganese oxidation, Frontiers of Environmental Science & Engineering (2022). DOI: 10.1007/s11783-023-1619-y