A team of researchers at the University of Illinois at Urbana-Champaign has identified a method for producing a certain type of molecule that could pave the way for new pharmaceuticals to treat diseases that are currently incurable.
If you open your medication cabinet, you will most certainly encounter organic ammonia derivatives known as amines. They are one of the most common structures discovered in pharmaceuticals today. More than 40% of medications and drug prospects contain amines, with 60% of those amines being tertiary, named for the three carbons bound to nitrogen.
Tertiary amines are found in a wide range of human pharmaceuticals, including antibiotics, breast cancer and leukemia meds, opioid pain relievers, antihistamines, blood thinners, HIV treatments, antimigraine medications, and others. They boost the solubility of a medication and can activate its critical biological effects.
Despite the prominence of this specific class of chemicals in medicine today, it is likely that most of the functional potential of tertiary amines remains untapped.
Because the usual method of producing them necessitates particular, controlled conditions, the identification of new tertiary amines, which could potentially treat a wide spectrum of currently untreatable disorders, is hampered.
“Because the conditions are so simple and work for so many different amines and olefins there is great potential to adopt this reaction for automation,”
White said
An Illinois research team led by M. Christina White, Lycan Professor of Chemistry, and graduate students Siraj Ali, Brenna Budaitis, and Devon Fontaine has discovered a new chemical reaction, a carbon-hydrogen amination cross-coupling reaction, that creates a faster, simpler way of producing tertiary amines without the inherent limitations of traditional methods. The researchers believe this might also be utilized to uncover new nitrogen reactions.
This new reaction in the chemist’s toolbox converts the traditional tertiary amine building process—with its classic chemical reactions requiring highly specialized conditions specific to each molecule—into a procedure that can be carried out in general conditions open to air and moisture and with the potential for automation.
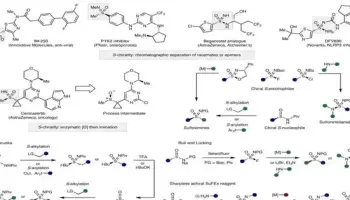
This new procedure, described by the researchers in their recently published paper in Science, uses a metal catalyst discovered by their group (Ma-WhiteSOX/palladium) and two building blocks—abundant hydrocarbons (olefins with adjacent C—H bonds) and secondary amines—to generate a variety of tertiary amines.
According to White, this has the potential for chemists to take a variety of different secondary amines and couple them to a variety of different olefins, both of which can be purchased or easily synthesized.
“And these are long-lasting beginning elements. You could have them in separate containers, mix and match them, and then use our catalyst to create a variety of tertiary amine combinations, “White stated. “The adaptability of this reaction facilitates the discovery of tertiary amine medicines.”
The difference between classical reactions and this new reaction for producing tertiary amines is like choosing a specialty sandwich from a menu versus designing your own sandwich from a wide collection of ingredients—you have a lot more options.
This very adaptable technique for producing tertiary amines is also extremely practical.
“You could, in theory, operate it on your stove top,” White says. You don’t need to take many precautions with it; you can run it open to the air and without excluding water. All you need are your starting ingredients, the palladium/SOX catalyst, and some heat. It should operate just as it does in the lab. “
White said that while a pharmaceutical business may need to use specialist processes to produce tertiary amines, this reaction allows you to take two simple, often commercial, starting ingredients and combine them using the same procedure.
Because the conditions are so basic and work for so many different amines and olefins, there is a lot of promise for automating this reaction, White says.
The scientists resolved a long-standing difficulty in C—H functionalization chemistry by replacing a hydrogen atom in a molecule’s carbon framework with a basic, secondary amine to directly produce tertiary amines.
Metal catalysts prefer to interact with basic amines rather than the olefin’s C—H bonds. The researchers anticipated that amine salts (easy-to-use and store amine-BF3 salts) could avoid this interaction with the catalyst.
The palladium/SOX catalyst, which acts like a dam to limit the flow of water, regulates the gradual release of amines from salts and mediates the coupling of the secondary amine and hydrocarbon to produce the tertiary amine product.
The researchers generated 81 tertiary amines in their investigation, demonstrating the potential of this new chemical reaction by linking a wide range of complex, medicinally important secondary amines to many complex olefins possessing reactive functionality. In the classic tertiary amine manufacturing procedures, this contains functionality that is reactive with secondary amines.
To demonstrate the potential for new medicines, the researchers used this new reaction to efficiently synthesize 12 existing drug compounds, including Abilify, an anti-psychotic medication, Naftin, an anti-fungal, and 11 complex drug derivatives, including the anti-depressants Paxil and Prozac, as well as Plavix, a blood thinner.
In addition to using this reaction in the pharmaceutical industry as a platform to accelerate the development of new tertiary amine medications, the researchers believe that their catalyst-controlled slow-release technique might be utilized by other researchers to discover many more new nitrogen reactions.