Scientists from the National Institutes of Health have fostered a three-layered structure that permits them to perceive how and where illness changes in the sparkle protein can prompt mitochondrial infections. The protein is involved in assisting cells with utilizing the energy our bodies convert from food. Before the advancement of this 3D design, analysts just had models and couldn’t decide how these changes added to illness. Mitochondrial illnesses are a group of acquired conditions that influence 1 out of 5,000 individuals and have few medicines.
“Interestingly, we can plan the changes that are causing some of these staggering illnesses,” said lead creator Amanda A. Riccio, Ph.D., and analyst in the National Institute of Environmental Health Sciences (NIEHS) Mitochondrial DNA Replication Group, which is essential for NIH. “Clinicians can now see where these changes lie and can utilize this data to assist with pinpointing causes and assist families with deciding, including choices about having more kids.”
“What is so lovely about Dr. Riccio’s and the team’s work is that the structure allows you to see so many of these disease mutations gathered in one location,”
Matthew J. Longley, Ph.D.
The new discoveries will be especially important for creating designated medicines for patients who experience the ill effects of mitochondrial sicknesses. For example, moderate outer ophthalmoplegia, a condition that can prompt loss of muscle capabilities engaged with eye and eyelid development; Perrault disorder, an uncommon hereditary issue that can cause hearing misfortune; puerile beginning spinocerebellar ataxia, an innate neurological problem; and hepatocerebral mitochondrial DNA (mtDNA) exhaustion disorder, a genetic infection that can prompt liver disappointment and neurological confusions during outset.
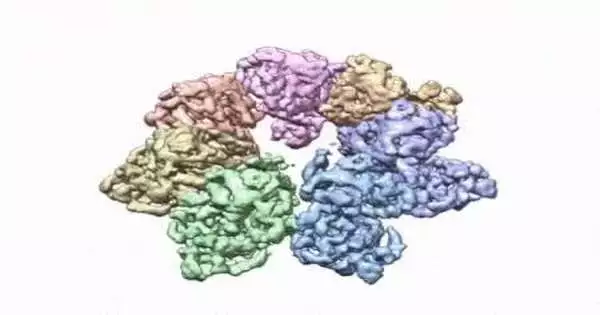
This turning picture shows the 3D design that NIEHS analysts made of the sparkle protein. The analysts utilized Cryo-EM and different methods to demonstrate the way that disease changes in the protein can prompt mitochondrial infections. The video zooms to the protein interface where large numbers of the illness’s changes happen. NIEHS credit: A.A. Riccio
The paper that shows up in the Proceedings of the National Academy of Sciences features how the NIEHS analysts were quick to precisely plan clinically important variations in the sparkle helicase, the protein that loosens up the mitochondrial DNA twofold helix. The sparkle design and every one of the directions are presently accessible in the open information protein data bank that is unreservedly accessible to all analysts.
“The design of sparkle has evaded analysts for a long time.” It’s a truly challenging protein to work with, “noted William C. Copeland, Ph.D., who drives the Mitochondrial DNA Replication Group and is the corresponding creator of the paper. “By settling the protein and involving the best gear on the planet, we had the option to assemble the last lacking part for the human mitochondrial DNA replisome.”
The analysts utilized cryo-electron microscopy (CryoEM), which permitted them to see inside the protein and the complex designs of many amino acids, or buildups, and how they connect.
Mitochondria, which are liable for energy creation, are particularly helpless against changes. mtDNA changes can upset their capacity to create energy effectively for the cell. In contrast to other specific designs in cells, mitochondria have their own DNA. In a cell’s core, there are two duplicates of every chromosome, but in the mitochondria there could be a great many duplicates of mtDNA. Having countless mitochondrial chromosomes permits the cell to endure a couple of changes, yet the gathering of too many transformed duplicates prompts mitochondrial illness.
To lead the review, the scientists utilized a clinical change, W315L, known to prompt moderate outer ophthalmoplegia, to tackle the design. Utilizing CryoEM, they had the option to notice a great many protein particles showing up in various directions. The last design shows a multi-protein round plan. They also utilized mass spectrometry to check the design and, afterward, did virtual experiences to comprehend why the change brings about illness.
Inside sparkle, they had the option to plan up to 25 illness-causing changes. They found that large numbers of these illness changes map right at the intersection of two protein subunits, proposing that transformations in this area would debilitate how the subunits connect and make the helicase unfit to work.
“The plan of sparkle is a lot like a riddle.” A clinical transformation can change the state of the sparkle pieces, and they may presently not fit together as expected to do the planned capability,” Riccio made sense of.
“What is the big deal about Dr. Riccio and the cooperation is that the design permits you to see so many of these illness changes gathered in one spot,” said Matthew J. Longley, Ph.D., a creator and NIEHS scientist. “It is strange to see one paper that makes sense of such countless clinical changes.” On account of this work, we are one bit nearer to having data that can be utilized to foster medicines for these crippling illnesses. “
More information: Amanda A. Riccio et al, Structural insight and characterization of human Twinkle helicase in mitochondrial disease, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2207459119
Journal information: Proceedings of the National Academy of Sciences