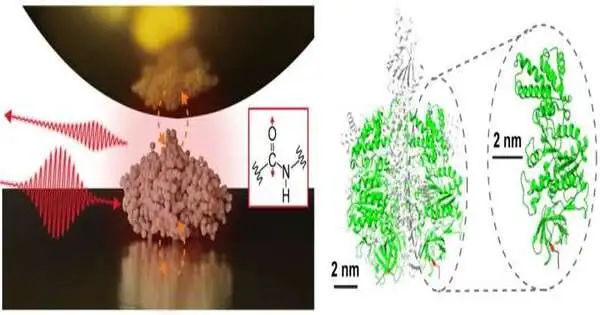
An interdisciplinary examination group, led by Colleague Prof. Jun Nishida and Partner Prof. Takashi Kumagai at the Establishment for Sub-atomic Science, has effectively noticed vibrational spectra of single proteins, comprising roughly 500 amino corrosive deposits, utilizing progressed estimation strategies in view of close field optical microscopy. This strategy uses light restricted at the nanometer scale, considering the definite investigation of tiny examples, which was formerly difficult with traditional infrared spectroscopy.
The review is distributed in the Nano Letters diary.
Ordinary infrared spectroscopy has been generally utilized for the underlying and synthetic investigation of different materials as it can gauge vibrational spectra, frequently alluded to as “atomic fingerprints.”
The new accomplishment addresses a significant progression towards mechanical developments like super-touchy and super-goal infrared imaging, as well as single-particle vibrational spectroscopy.
The fast improvement of nanotechnology as of late has prompted an expanding interest in super-high responsiveness and super-goal infrared imaging. Notwithstanding, customary infrared spectroscopy is restricted to estimating minuscule examples or accomplishing nanometer-scale spatial goals. For instance, even infrared microspectroscopy with great responsiveness expects more than 1,000,000 proteins to get into the infrared range, making it difficult to quantify only a solitary protein.
In their review, the exploration group disengaged a solitary protein, a sub-unit containing a protein complex called F1-ATPase, on a gold substrate and performed close-field infrared spectroscopy estimations in an encompassing climate.
They effectively procured the infrared vibrational range of a solitary protein, addressing a serious step forward that might prompt portraying neighborhood primary associations of individual proteins. Such data is especially significant for figuring out the modern elements of protein buildings and film proteins, offering further insights into their components and connections.
Moreover, they have fostered another hypothetical structure depicting the nanoscale collaborations between the infrared close to the field and protein.
In view of the hypothesis, the group had the option to quantitatively replicate the trial vibrational spectra that they noticed. These outcomes will be important for the synthetic investigation of biomolecules as well as different nanomaterials, preparing for the scope of uses of nanoscale infrared spectroscopy.
More information: Jun Nishida et al, Sub-Tip-Radius Near-Field Interactions in Nano-FTIR Vibrational Spectroscopy on Single Proteins, Nano Letters (2024). DOI: 10.1021/acs.nanolett.3c03479