A semiconductor is a material whose conductivity lies somewhere close to that of a guide and an encasing. This property permits semiconductors to act as the basis material for present-day gadgets and semiconductors. It is no exaggeration to say that the semiconductor industry was largely responsible for mechanical advancement in the latter half of the twentieth century.
Today, mechanical headways in semiconductor nanocrystals are, as of now, progressing. For instance, quantum specks and wires from semiconducting materials are of extraordinary interest in displays, photocatalytics, and other electronic gadgets. Nonetheless, various parts of the colloidal nanocrystals presently can’t seem to be perceived at the basic level. A significant one among them is the clarification of the sub-atomic level components of the development and development of nanocrystals.
These semiconducting nanocrystals are developed starting from minuscule individual forerunners made of a few iotas. These antecedents are called nanoclusters. Confinement and sub-atomic design assurance of such nanoclusters (or basically groups) have been the subject of enormous interest for a very long time. The primary subtleties of bunches, ordinarily the cores of the nanocrystals, are expected to give fundamental experiences into the advancement of the nanocrystals’ properties.
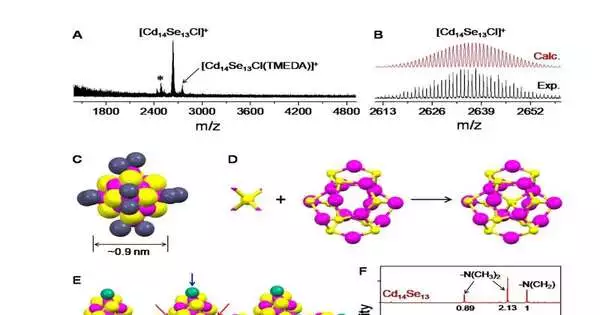
“We discovered that the tertiary diamine and halocarbon solvent are critical in producing nearly single-sized, stoichiometric clusters.” The tertiary diamine ligands (N,N,N,”N’-tetramethylethylenediamine) not only provide rigid binding with appropriate steric constraints, but also disable intercluster interactions due to the short carbon chain, resulting in the formation of soluble Cd14Se13 clusters rather than undesirable insoluble lamellar Cd13Se13 assemblies.”
Director Hyeon
Unique “seed” nanoclusters bring about the development of various nanocrystals. Thusly, it is vital to have a homogenous combination of indistinguishable nanoclusters in the event that one wishes to develop them. Notwithstanding, the union of nanoclusters frequently brings about the development of bunches of various sizes and arrangements, and decontaminating the combination to get just the beneficial particles is exceptionally difficult.
“Sorcery estimated nanoclusters, MSCs,” which are ideally shaped over irregular sizes in a uniform way, have a size range of 0.5 to 3.0 nm. Among these, MSCs made out of non-stoichiometric cadmium and chalcogenide proportion (non 1:1) are the most contemplated. Another class of MSCs with a 1:1 stoichiometric proportion of metal-chalcogenide proportion has been under the spotlight, inferable from the forecast of fascinating designs. For instance, Cd13Se13, Cd33Se33, and Cd34Se34, which comprise an equivalent number of cadmium and selenium particles, have been combined and described.
As of late, scientists at the Center for Nanoparticle Research (drove by Professor Hyeon Taeghwan) inside the Institute for Basic Science (IBS) in a joint effort with the groups at Xiamen University (drove by Professor Nanfeng Zheng) and at the University of Toronto (drove by Professor Oleksandr Voznyy) revealed the colloidal combination and nuclear level design of stoichiometric semiconductor cadmium selenide (CdSe) bunch. This is the smallest nanocluster blended starting today.
The blend of Cd14Se13 was achieved after various past disappointments with Cd13Se13, which generally wound up in bothersome congregations, making them difficult to describe. Chief Hyeon expressed, “We tracked down that the tertiary diamine and halocarbon dissolvable assume an urgent part in accomplishing almost single-sized, stoichiometric groups.” The tertiary diamine (N,N,N) ligands not just give unbending, restricting suitable steric imperatives, but in addition debilitate the intercluster communications because of the short carbon chain, prompting the arrangement of dissolvable Cd14Se13 groups, rather than undesired insoluble lamellar Cd13Se13 congregations. “
The dichloromethane dissolvable supplies chloride particles in situ to at the same time accomplish charge-adjusting of the fourteenth cadmium particle, which considers the self-gathering of the bunches to frame (Cd14Se13Cl2)n. Thus, single gems of satisfactory quality could be gotten for the scientists to decide their design. The pieces of the bunches got from the single precious stone X-beam diffraction information examination were generally excellent in concurrence with the mass spectrometry and atomic attractive reverberation information. The general state of the bunch was circular, with a size of around 0.9 nm.
While most different MSCs with non-1:1 metal-chalcogenide proportions will generally have supertetrahedral math, the new Cd14Se13 was found to have a center enclosure plan of constituent iotas. In particular, the group contained a focal Se iota typified by a Cd14Se12 confine with an adamantane-like CdSe plan. Such a novel plan of molecules opens the chance of developing nanocrystals with surprising designs, which should be investigated further from now on.
The optical properties of the bunch showed the presence of quantum-constraint impacts with band-edge photoluminescence. Notwithstanding, the photoluminescence highlights connected with deformity states were noticeable because of the tiny size of the groups. The design and the retention tops seen in the examinations were very much upheld by the thickness utilitarian hypothesis computations.
The scientists made the Cd14Se13 bunch through a halfway Cd34Se33 group, which is the next known huge measured stoichiometric group. Curiously, both of these two bunches could be doped through replacement with a limit of two Mn iotas, which shows the possibility to weaken attractive semiconductors with custom-made photoluminescence properties. The computational outcomes showed that the Cd destinations bound to halides were more defenseless to Mn replacement.
The ramifications of this study might work out positively past the combination of single-sized semiconductor groups, as the tertiary diamines of various compound designs might be stretched out to different bunches. Amalgamation and assurance of the nuclear level design of different groups may ultimately assist with understanding the sub-atomic level development component of the semiconductor nanocrystals.
It was shown that the Cd34Se33 bunch could be dynamically settled through a ligand-trade prompted size transformation process created in this work. However, more efforts and new techniques are expected to further develop the arrangement state steadiness for the design assurance of the following huge estimated bunch Cd34Se33, which is the fundamental core for the development of cadmium selenide-based nanocrystals. It is believed that further investigations of the size-, structure-, and dopant-conditions on the optoelectronic, photocatalytic, and spintronic applications might yield new bearings to logical examination of the semiconductor groups.
More information: Taeghwan Hyeon, Structure of a subnanometer-sized semiconductor Cd14Se13 cluster, Chem (2022). DOI: 10.1016/j.chempr.2022.06.025. www.cell.com/chem/fulltext/S2451-9294(22)00330-8
Journal information: Chem