Hydrogen (H2) is right now being talked about as an optimal energy transporter of sustainable power sources. Hydrogen has the most elevated gravimetric energy thickness of every substance fuel (141 MJ/kg), which is multiple times higher than gas (46 MJ/kg). In any case, its low volumetric thickness confines its far and wide use in transportation applications—as current stockpiling choices require a ton of room.
At its surrounding temperature, hydrogen is a gas, and one kilogram of hydrogen involves a volume of 12000 liters (12 cubic meters). In energy-component vehicles, hydrogen is put away under an exceptionally high tension of multiple times the environmental strain, which reduces the volume to 25 liters for every kilogram of H2. Fluid hydrogen shows a higher thickness, bringing about 14 liters for every kilogram, except it requires incredibly low temperatures since the edge of boiling over of hydrogen is only 253 °C.
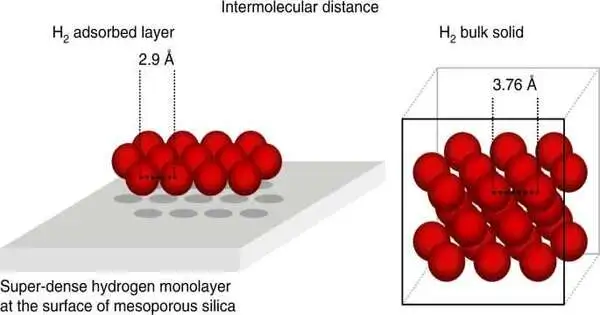
Presently, a group of researchers from the Max Planck Institute for Intelligent Systems, the Technische Universität Dresden, the Friedrich-Alexander-Universität Erlangen-Nürnberg, and Oak Ridge National Laboratory has exhibited that hydrogen consolidates on a surface at an exceptionally low temperature close to the H2 edge of boiling over, framing a super-thick monolayer surpassing the thickness of fluid hydrogen by a component of very nearly three, which decreases the volume to just 5 liters for each kilogram of H2.
The amazing outcome was that two times as numerous H2 particles as molecules of the honorable gas argon covered the surface, despite the fact that both have almost the same size. H2 particles crush intently together, forming a super-thick layer, to double the number of particles per region.
The concentrate by R. Balderas-Xicohténcatl et al. involved high-goal cryo-adsorption investigating profoundly requested mesoporous silica showing obvious pore and surface qualities to decide the quantity of particles consolidated on the material’s surface.
Inelastic neutron dissipation is an optimal device to follow the arrangement of this two-layered hydrogen layer. Interestingly, the presence of this super-thick hydrogen was affirmed in situ. This immediate perception was only possible with the high-goal neutron vibrational spectrometer VISION, which has an inelastic count rate that is multiple times higher than any comparable accessible spectrometer.
Hypothetical examinations affirm the exploratory perceptions of the uncommonly high hydrogen thickness in the adsorbed layer. The appealing powers at the surface were more grounded than the repugnance between two hydrogen particles, bringing about a super-thick hydrogen pressing on the mesoporous silica surface. The super-high thickness is an outcome of the great compressibility of hydrogen, which doesn’t have any center electrons.
The development of the super-thick hydrogen layer at low temperatures close to the limit is of crucial interest. It ought to be considered for quantitative investigation of H2 adsorption isotherms at 20 K. It might also provide additional opportunities for improving the volumetric limit of cryogenic hydrogen stockpiling frameworks for some applications in an approaching hydrogen economy.
The exploration was published in Nature Chemistry.
More information: Rafael Balderas-Xicohténcatl et al, Formation of a super-dense hydrogen monolayer on mesoporous silica, Nature Chemistry (2022). DOI: 10.1038/s41557-022-01019-7
Journal information: Nature Chemistry