A group from the UPC and the Catalan Organization of Nanoscience and Nanotechnology (ICN2) has planned an effective and stable photocatalyst fit for creating hydrogen straightforwardly utilizing daylight. The outcomes are distributed in the diary, Nature Correspondences.
Hydrogen is fundamental for that energy progress, however long it is delivered from inexhaustible sources (green hydrogen). It has for some time been realized that electrons in certain semiconductors can take part in synthetic responses when enlightened by daylight.
This is the situation with titanium dioxide, a modest and innocuous material that is broadly utilized as a white color in paints, plastics, papers, inks, and beauty care products. The energized electrons in titanium dioxide are equipped to produce hydrogen from the protons in water and natural mixtures. Nonetheless, hydrogen creation is extremely low in light of the fact that the electrons will generally unwind as opposed to respond, so the effectiveness of the cycle is excessively low according to a pragmatic perspective.
This impediment can be overcome by carrying titanium dioxide into contact with metal nanoparticles, which act as electron channels, broadening the existence of the electrons in an energized state so they can respond and create hydrogen. This permits us to accomplish many times more significant returns.
This study is a step in the right direction for maintainable hydrogen creation. It was driven by Ramón y Cajal scientist Lluís Soler and teacher Jordi Llorca from the Reprise NEMEN research gathering of the Branch of Compound Designing and the Establishment of Energy Advances of the Universitat Politècnica de Catalunya—BarcelonaTech (UPC). They are likewise essential for the Particular Community for Hydrogen Exploration (CER-H2).
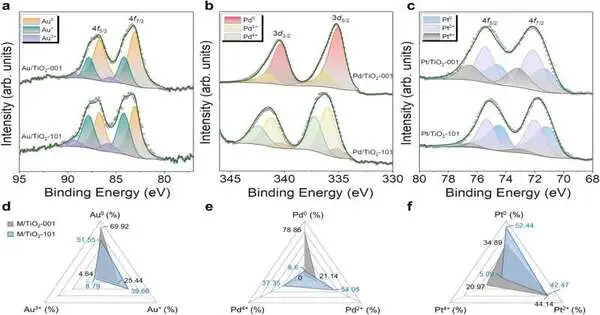
Utilizing a mechanochemical cycle, the specialists stored metal bunches on titanium dioxide nanoparticles of different morphologies and found that the different uncovered crystallographic countenances of titanium dioxide likewise assume a key role in hydrogen creation. Both the security of photocatalysts and the strength of electron movement between the semiconductor and the metal nanoparticles are emphatically connected with the semiconductor’s uncovered faces, which are responsible for iota versatility and conglomeration.
The outcomes are clear. At the point when platinum groups are stored on octahedral titanium dioxide nanoparticles, a photocatalyst is obtained that produces higher amounts of hydrogen and, all the more significantly, is considerably more steady than some other mix. The review is a wonderful illustration of how nanotechnology can be applied to plan new gadgets in the field of energy.
To comprehend the outcomes, Ramón y Cajal analyst Claudio Cazorla from the UPC’s Branch of Physical Science has done quantum mechanical computations to concentrate on the electronic design of the photocatalysts, which were contrasted, and the consequences of X-beam photoelectron spectroscopy acquired at the UPC’s Exploration Place in Multiscale Science and Designing. The middle is situated on the Inclining Besòs Grounds, just like the Barcelona East School of Designing (EEBE), where the specialists additionally instruct.
The results of this examination will empower the development of new impetuses for the proficient and economical creation of green hydrogen. As of now, work is in progress at the UPC’s Particular Place for Hydrogen Exploration to try these outcomes.
More information: Yufen Chen et al, Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution, Nature Communications (2023). DOI: 10.1038/s41467-023-41976-2