Metals are ordinarily utilized as dynamic materials for negative terminals in batteries. Rechargeable metal-air batteries with oxygen-reducing positive electrodes have recently utilized redox-active organic molecules like molecules based on quinones and amines as negative electrodes. Hydroxide ions and protons take part in the redox reactions here. These batteries have high performance and are close to their theoretical maximum capacity.
Additionally, the formation of structures known as “dendrites,” which have a negative impact on battery performance and the environment, can be avoided when redox-active organic molecules are used in rechargeable air batteries to overcome issues that are associated with metals. However, unlike metal-based batteries, these batteries make use of liquid electrolytes, which pose significant safety issues such as flammability, leaching, and high electrical resistance.
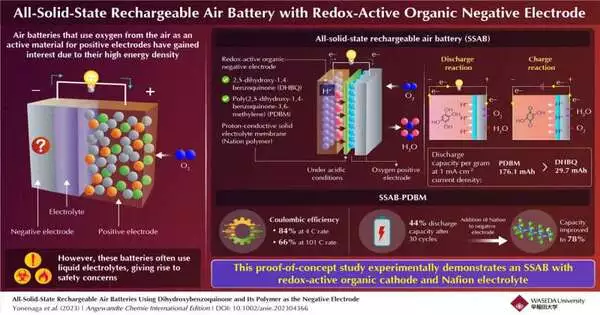
A group of Japanese researchers have developed an all-solid-state rechargeable air battery (SSAB) and investigated its capacity and durability in a recent study that was published in the Angewandte Chemie International Edition. Professor Kenichi Oyaizu from Waseda University co-authored the study, which was led by Professor Kenji Miyatake from Waseda University and the University of Yamanashi.
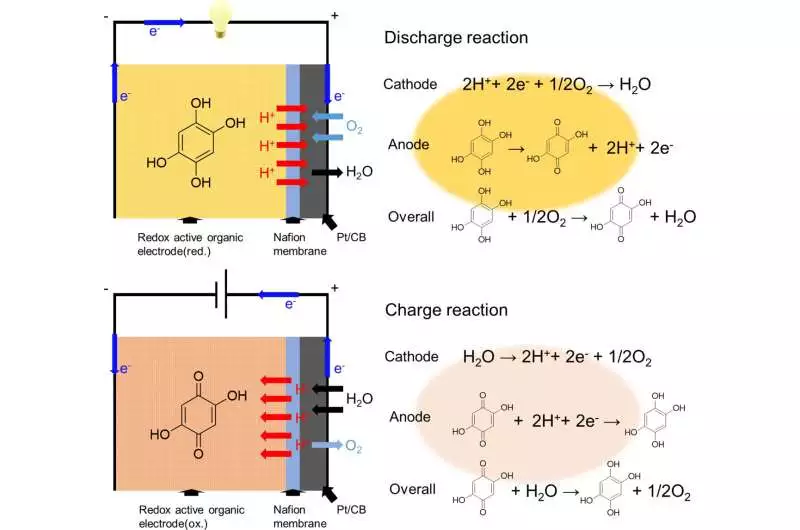
Due to their stable and reversible redox reactions in acidic conditions, the researchers selected 2,5-dihydroxy-1,4-benzoquinone (DHBQ) and its polymer, poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene) (PDBM), as the active materials for the negative electrode. They also used Nafion, a proton-conductive polymer, as the solid electrolyte instead of traditional liquid electrolytes.
An all-solid-state rechargeable air battery with a Nafion polymer electrolyte and organic negative electrode based on dihydroxybenzoquinone has been created by researchers. Credit: Kenji Miyatake from Waseda University.
“To the best of my knowledge, no air batteries based on organic electrodes and solid polymer electrolytes have been developed yet,” says Kenji Miyatake.
The experimental evaluation of the SSAB’s charge-discharge performance, rate characteristics, and cyclability followed its installation. They discovered that the SSAB did not deteriorate in the presence of water and oxygen, in contrast to conventional air batteries, which have an organic liquid electrolyte and a metallic negative electrode.
In addition, PDBM, a polymer of the redox-active molecule DHBQ, produced a superior negative electrode. At a constant current density of 1 mAcm2, the SSAB-PDBM had a capacity of 176.1 mAh, whereas the SSAB-DHBQ had a capacity of 29.7 mAh per gram of discharge.
The coulombic efficiency of SSAB-PDBM was also found to be 84% at a rate of 4 C, but gradually decreased to 66% at a rate of 101°C, according to the researchers. Although the discharge capacity of SSAB-PDBM decreased to 44% after 30 cycles, the researchers were able to significantly increase it to 78% by increasing the proportion of proton-conductive polymer in the negative electrode. The addition of Nafion increased the PDBM-based electrode’s performance and durability, as demonstrated by electron microscopic images.
An SSAB with a negative electrode made of redox-active organic molecules, a solid electrolyte made of a proton-conducting polymer, and a positive electrode made of oxygen-reducing diffusion type is shown to work well in this study. The researchers anticipate that it will open the door to future developments. Miyatake draws a conclusion by stating that “this technology can extend the battery life of small electronic devices such as smartphones and eventually contribute to the realization of a carbon-free society.”
More information: Makoto Yonenaga et al, All‐Solid‐State Rechargeable Air Batteries Using Dihydroxybenzoquinone and Its Polymer as the Negative Electrode, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202304366