Glucocorticoids, like cortisone, are among the most commonly used sedatives, and are used to treat asthma, psoriasis, organ transplantation, and even Coronavirus.As to pharmacological activity, the action of the glucocorticoid receptor (GR) is vital.
The GR is a record factor that regulates critical human physiological cycles.In any case, the itemized, three-layered design of this atomic receptor—perhaps the main objective in the drug business—is as yet a riddle to established researchers.
Presently, a review distributed in the journal Nucleic Acids Exploration uncovers interestingly that GR is a profoundly plastic protein with an exceptionally flexible design: its monomers (constituent particles) can self-assemble in various ways to frame dimers, tetramers, and edifices with different proteins in the cell core to control the outflow of various qualities.
The discovery of the previously unknown primary and useful flexibility of the GR and its atomic self-gathering process (oligomerization) will add to the plan of medications that are more specific with the specific compliances of the receptor, as well as less harmful to avoid the serious side effects that traditional corticosteroids cause in patients.
“Our research investigates the oligomerization capability of GR-LBD and demonstrates that this receptor can generate up to 20 distinct dimers. When the receptor attaches to DNA, some of these dimers may create functional tetramers, according to the findings.”
Eva Estébanez, from the Department of Biochemistry and Molecular Biomedicine of the Faculty of Biology.
Staying away from the results of glucocorticoids
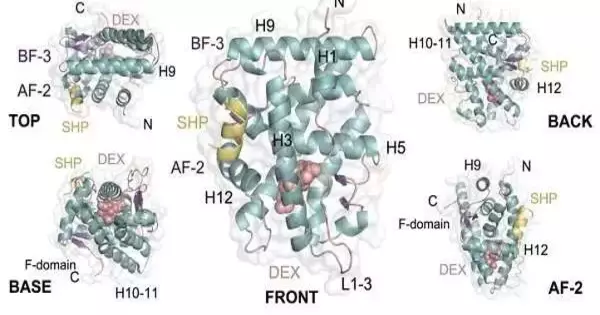
The three-layered design of the GR, which is fundamental to its physiological action, has been addressed in the logical writing. The main design of the GR ligand-restricting area (GR-LBD) was distributed in 2002 in the journal Cell. As per this model, two GR-LBD particles partner to frame a dimer in a conformation that until recently was never depicted in atomic receptors.
These discoveries opened a logical discussion—wwhich actually exists—oon the conformity of the RBC and its oligomerization state in cells. Since drug organizations have been quick to foster medications against the GR, the majority of the ensuing primary examinations have zeroed in on the connection of the GR-LBD with helpful mixtures. Thus, the examination of the oligomerization province of RBC was ignored, creating a lot of primary information that remained unexamined exhaustively.
Research on glucocorticoid activity without aftereffects has been founded solely on this halfway model of the GR dimerization state. Generally, the GR, once enacted by corticosteroids, was considered to be able to carry out various roles in the cell depending upon its oligomerization state: as a monomer, it curbed favorable to fiery qualities, while as a dimer, it could prompt the declaration of calming qualities.
This hypothesis was tested when the NIH group in Bethesda demonstrated that the GR could also function as a tetramer (four GR particles linked together, possibly a dimer of dimers) and have physiological action, whereas the monomeric form of the receptor had no capability.
Nonetheless, the data that was collected had some significant awareness of the design of the GR but couldn’t make sense of how the receptor shapes these tetramers at the cell level. “Our work examines the oligomerization capability of GR-LBD and demonstrates the way that this receptor can frame up to 20 unique dimers.” “The outcomes propose that a portion of these dimers can partner to frame useful tetramers when the receptor ties to DNA,” says Eva Estébanez, from the Branch of Organic Chemistry and Sub-atomic Biomedicine of the Faculty of Science.
The concentrate likewise recognized non-useful hexameric types of GR freaks that have been depicted in patients who don’t respond to corticosteroids (Chrousos disorder or glucocorticoid opposition condition). “As a result, our review partners intriguingly the development of non-useful oligomers of GR (or of some other atomic receptor) to a rare human endocrinological illness of glucocorticoid opposition,” Estébanez says.
An obscure primary pliancy in other atomic receptors
To get the outcomes, the group has applied many strategies, from X-beam crystallography with synchrotron radiation (ALBA-CELLS) to the strategy known as “number and splendor,” a main microscopy method that permits perception of the oligomerization province of RBCs in living cells.
The review has permitted the scientists to make sense of, according to a primary perspective, how GR dimers and tetramers can be shaped and how the ligand-restricting space is vital to these various compliances. The examination of the multitude of primary information accessible for the GR, along with the new designs tackled by the UB-IBUB bunch, has permitted them to determine an underlying pliancy never seen before in other atomic receptors.
“This flexibility permits the RBC to shape dimers with various compliances that can be tweaked, somewhat contingent upon the kind of ligand that ties to the receptor, and this would clarify the capacity of the RBC to structure tetramers,” says analyst Alba Jiménez.
“Our findings support the information demonstrating the arrangement of dynamic tetramers when the receptor binds to DNA and combine the speculation that the GR’s system of activity in the guideline of record is significantly more perplexing and flexible,” says master Andrea Alegre.
This multidisciplinary approach has made it conceivable to move the outcomes from perceptions gained from protein design to processes happening at the cell level, a logical advancement with ramifications for interest in human physiology and the battle against specific illnesses.
More information: Alba Jiménez-Panizo et al, The multivalency of the glucocorticoid receptor ligand-binding domain explains its manifold physiological activities, Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac1119
Journal information: Nucleic Acids Research