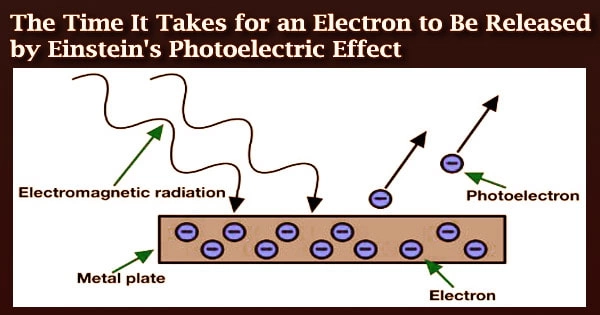
Albert Einstein received the Nobel Prize in Physics for his studies on the photoelectric effect one hundred years ago. Einstein had also carried out groundbreaking research on the photoelectric effect, albeit the jury had not yet fully grasped his ground-breaking theory of relativity.
He was able to show through analysis that light is made up of distinct energy packets known as photons. Max Planck’s notion that light is made up of quanta was conclusively confirmed by this, paving the path for contemporary quantum theory.
Despite the fact that the photoelectric effect in molecules has been extensively investigated in the past, it has not yet been possible to evaluate how it changes over time in an experimental setting.
How long does it take for an electron to be ejected in a particular direction after a light quantum strikes a molecule?
The quantum world of atoms and particles is renowned for being extraordinarily weird, with phenomena like existing in two locations at once and tunneling through impassable barriers. However, the peculiarities of quantum mechanics are not artifacts of mathematics; rather, they are actual phenomena that have been repeatedly observed in lab settings.
“The length of time between photon absorption and electron emission is very difficult to measure because it is only a matter of attoseconds,” explains Till Jahnke, the PhD-supervisor of Jonas Rist. This corresponds to just a few light oscillations. “It has so far been impossible to measure this duration directly, which is why we have now determined it indirectly.”
Entanglement, which describes particles that are inexplicably linked regardless of their distance from one another, is one of the most recognizable aspects of quantum mechanics. However, it is proving challenging to fulfill the promises of entanglement-based technologies. This is due to the fragility of the entanglement phenomena.
Individual pairs of particles are often created during entanglement experiments. Single particles, however, are challenging to precisely detect and are frequently lost or masked by surrounding noise.
The length of time between photon absorption and electron emission is very difficult to measure because it is only a matter of attoseconds. This corresponds to just a few light oscillations. It has so far been impossible to measure this duration directly, which is why we have now determined it indirectly.
Till Jahnke
The researchers achieved this using a COLTRIMS reaction microscope, a measurement tool that allows for an incredibly detailed examination of individual atoms and molecules.
A sample of carbon monoxide was placed in the middle of the reaction microscope, and the researchers used the synchrotron radiation source BESSY II of Helmholtz-Zentrum Berlin to produce exceptionally powerful X-ray light.
One oxygen and one carbon atom make up the carbon monoxide molecule. Now that the X-ray beam had the ideal amount of energy, it was able to knock one electron out of the carbon atom’s innermost electron shell.
The effect is that the molecule breaks up. The emitted electron and oxygen and carbon atoms were then measured.
“And this is where quantum physics comes into play,” explains Rist. “The emission of the electrons does not take place symmetrically in all directions.”
Due to the outstanding axis of carbon monoxide molecules, the electrostatic fields of the molecule continue to effect the expelled electrons as long as they are still close by. Depending on which direction the electrons are ejected, this delays the release to varying degrees.
Since electrons follow the rules of quantum physics, they have both a particle and a wave nature, which ultimately emerges as an interference pattern on the detector.
“On the basis of these interference effects, which we were able to measure with the reaction microscope, the duration of the delay could be determined indirectly with very high accuracy, even if the time interval is incredibly short,” says Rist. “To do this, however, we had to avail of several of the possible tricks offered by quantum physics.”
On the one hand, the results demonstrated that the electron is indeed emitted in just a few dozen attoseconds. However, they also showed that the direction in which the electron exits the molecule and the velocity of the electron both have a significant impact on the time interval and the emission time, respectively.
These measurements are intriguing for more than just physics fundamental study. The models that are used to represent this kind of electron dynamics are applicable to a wide range of chemical events in which electrons are not completely freed but are transported to nearby molecules, for example, where they start new reactions.
“In the future such experiments could also help to better understand chemical reaction dynamics therefore,” says Jahnke.