Glucose is the sugar we ingest from the food varieties we eat. This fuel controls each cell in our bodies. Might glucose, at any point, additionally drive the upcoming clinical inserts?
Engineers at MIT and the Technical University of Munich suspect as much. They have planned another sort of glucose power module that changes glucose straightforwardly into power. The device is smaller than other proposed glucose power devices, measuring only 400 nanometers thick, or about one-tenth the width of a human hair.The sweet power source creates around 43 microwatts per square centimeter of power, accomplishing the most powerful thickness of any glucose energy unit to date under encompassing circumstances.
The new device is also tough, withstanding temperatures of up to 600 degrees Celsius.Whenever integrated into a clinical embed, the energy unit could stay stable through the high-temperature disinfection process expected for every single implantable gadget.
The core of the new gadget is produced using fire, a material that holds its electrochemical properties even at high temperatures and small scopes. Scientists imagine the new plan could be made into ultrathin movies or coatings and folded over inserts to supply inactively powered gadgets, utilizing the body’s plentiful glucose.
“Glucose is wherever in the body, and the thought is to reap this promptly accessible energy and use it to drive implantable gadgets,” says Philipp Simons, who fostered the plan as a feature of his Ph.D. proposition in MIT’s Department of Materials Science and Engineering (DMSE). “In our work we show another glucose power module electrochemistry.”
Rather than utilizing a battery, which can take up 90% of an embed’s volume, you could make a gadget with a meager film, and you’d have a power source with no volumetric impression, says Jennifer L. M. Rupp, Simons’ postulation boss and a DMSE visiting teacher, who is likewise an academic administrator of strong state electrolyte science at Technical University Munich in Germany.
“The goal is to gather this easily available energy and use it to power implantable devices, We demonstrate a new glucose fuel cell electrochemistry in our work.”
Philipp Simons, who created the concept as part of his Ph.D. thesis in MIT’s Department of Materials Science and Engineering (DMSE).
Simons and his associates detail their plan in the Advanced Materials diary. Co-creators of the review include Rupp, Steven Schenk, Marco Gysel, and Lorenz Olbrich.
A ‘hard’ separation
The motivation for the new energy component came in 2016, when Rupp, who has practical experience in ceramics and electrochemical gadgets, went to take a standard glucose test close to the furthest limit of her pregnancy.
“In the specialist’s office, I was an extremely exhausted electrochemist, figuring how you could manage sugar and electrochemistry,” Rupp recalls. “Then, at that point, I understood that it would be great to have a glucose-controlled strong state gadget.” Furthermore, Philipp and I met over espresso and worked out on a napkin the primary drawings. “
The group isn’t quick to think about a glucose power module, which was first presented during the 1960s and showed potential for changing glucose’s compound energy into electrical energy. In any case, glucose energy units at the time depended on delicate polymers and were immediately obscured by lithium-iodide batteries, which would become the standard power hotspot for clinical inserts, most eminently the heart pacemaker.
Nonetheless, batteries have a cutoff to how little they can be made, as their design requires the actual ability to store energy.
“Power modules straightforwardly convert energy instead of putting it away in a gadget, so you needn’t bother with everything necessary to store energy in a battery,” Rupp says.
Lately, researchers have looked again at glucose energy units as possibly more modest power sources, energized directly by the body’s bountiful glucose.
A glucose power module’s fundamental plan consists of three layers: a top anode, a center electrolyte, and a base cathode. The anode responds to glucose in natural liquids, changing the sugar into gluconic corrosive. This electrochemical change delivers a couple of protons and a couple of electrons. The center electrolyte acts to isolate the protons from the electrons, leading the protons through the energy component, where they join with air to shape particles of water—an innocuous result that streams away with the body’s liquid. In the meantime, the segregated electrons stream to an outer circuit, where they can be utilized to drive an electronic gadget.
The group hoped to develop existing materials and plans by changing the electrolyte layer, which is frequently produced using polymers. Polymer properties, in addition to their ability to lead protons, effectively corrupt at high temperatures, are difficult to maintain when downsized to the element of nanometers, and are difficult to disinfect.The researchers wondered if a ceramic—a highly safe material that can normally lead protons—could be made into an electrolyte for glucose energy units.
“At the point when you consider ceramics for such a glucose energy component, they enjoy the benefit of long-haul dependability, little versatility, and silicon chip combination,” Rupp notes. “They’re hard and strong.”
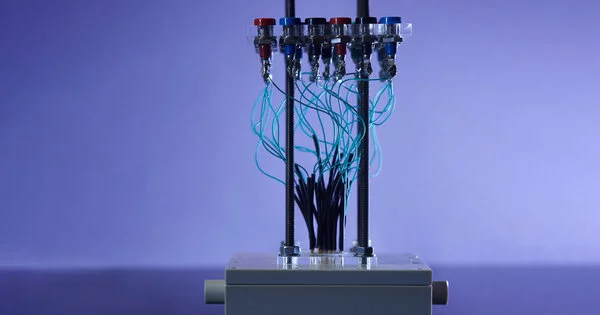
Image Credit: Kent Dayton
A “custom trial arrangement” is used to describe 30 glucose power devices in fast succession. Kent Dayton is the photographer.
Peak power
The analysts planned a glucose energy component with an electrolyte produced using ceria, a fired material that has high particle conductivity, is precisely vigorous, and, accordingly, is broadly utilized as an electrolyte in hydrogen power devices. It has additionally been demonstrated to be biocompatible.
“Ceria is effectively concentrated in the malignant growth research local area,” Simons notes. “It’s likewise like zirconia, which is utilized in tooth embeds and is biocompatible and safe.”
The group sandwiched the electrolyte with an anode and cathode made of platinum, a steady material that promptly responds to glucose. They created 150 individual glucose power devices on a chip, each around 400 nanometers flimsy and around 300 micrometers wide (about the width of 30 human hairs). They designed the cells on silicon wafers, demonstrating the way that the gadgets can be matched with a typical semiconductor material. They then, at that point, estimated the ongoing created by every cell as they streamed an answer of glucose over every wafer in an exclusively manufactured test station.
They found numerous cells created a pinnacle voltage of around 80 millivolts. Given the small size of every cell, this result is the most powerful thickness of any current glucose energy component plan.
“Excitingly, we can draw power and current that is adequate to control implantable gadgets,” Simons says.
It is the first occasion when proton conduction in electroceramic materials can be utilized for glucose-to-drive change, characterizing another kind of electrochemistry, Rupp says. “It expands the material use-cases from hydrogen energy components to very interesting glucose-change modes.”
The specialists “have opened another course to smaller than expected power hotspots for embedded sensors and perhaps different capacities,” says Truls Norby, a teacher of science at the University of Oslo in Norway, who didn’t add to the work. “The ceramics utilized are nontoxic, modest, and not least dormant both to the circumstances in the body and to states of cleansing preceding implantation.” The idea and show so far are promising, to be sure.





