Because of their minimal expense and ecological agreeableness, fluid zinc batteries can possibly assume a significant part in future energy stockpiling frameworks for applications like power matrices. However, a health concern has slowed the advancement of this emerging innovation.
In a July 28 review distributed in Nano Research, Chinese scientists introduced an answer that includes synthetically changing normal table sugar to balance out the zinc particle climate and secure future applications.
From electric vehicles to wind and solar power frameworks, an inexorably diverse range of force-hungry applications continues to support requests for large scope, low-cost energy capacity.As per the review, fluid zinc (Zn) batteries immediately rose to the top as one of the additional promising choices for economically satisfying the need.
“They are more safe and cost-effective than current lithium-ion batteries with flammable organic electrolytes, and the Zn anode has a super high theoretical capacity, making these Zn batteries even more promising for applications such as future grid energy storage.”
Meinan Liu, associate professor of nano-tech and nano-bionics at the University of Science and Technology of China
“They are high security and practical compared with current lithium-particle batteries with combustible natural electrolytes,” said paper writer Meinan Liu, academic administrator of nano-tech and nano-bionics at the University of Science and Technology of China. Furthermore, the Zn anode presents a high hypothetical limit, which makes these Zn batteries much better for applications like future framework energy capacity.
Nonetheless, when the zinc particle (Zn2+) focus on the outer layer of the anode drops to nothing, dendrites begin developing. Uncontrolled Zn dendrite development weakens electrochemical execution and represents a serious danger to safe activity.
“These dendrites can infiltrate the separator and prompt the battery to hiccup,” he said.
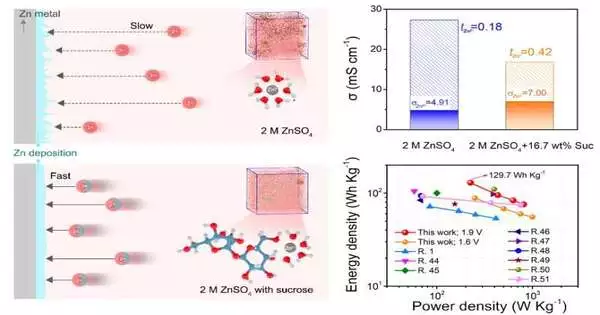
Past examinations have shown that changing the dissolvable climate (called “solvation structure”) can increase the portability of Zn2+ because the electric field effectively smothers the development of dendrites. The issue was that these past changes — like presenting different salts or including fewer water particles — wound up diminishing the ionic conductivity of the framework also.
There was a principal understanding hole between Zn2+ solvation structure and its portability, made sense of by Liu. This was a key variable influencing the dendrite development and soundness of the Zn anode.
In an endeavor to overcome this issue, a cooperative examination group from various Chinese establishments attempted another tack: presenting normal table sugar with numerous hydroxyl gatherings (a hydrogen and an oxygen bound together) into the electrolyte to change the solvation design of Zn2+.
By leading atomistic recreations and examinations, the exploration group confirmed that the sucrose particles upgraded portability and halted dendrite development without compromising dependability. This technique gave unlooked-to benefits too, truth be told:
“Discoveries affirm that sucrose particles in the solvation sheath not just upgrade the versatility, guaranteeing quick Zn2+ energy, but in addition shield the Zn anode from water erosion and effectively accomplish Zn sans dendrite affidavit and side response concealment,” Liu said.
This exhibits the incredible capability of involving this basic sucrose-change for future elite execution zinc batteries and brings the exploration field a bit nearer to the definitive objective of accomplishing a protected, green, superior presentation Zn battery.
“Ideally, this protected, minimal-expense Zn battery could be applied in framework energy capacity,” Liu said.
This method likewise fits extra varieties and changes: Zn-carbon cells convey higher energy thickness and further developed solidness, recommending an incredible expected use of sucrose-adjusted electrolytes for future Zn batteries.
In the ongoing examinations, the specialists will likewise be thinking about conceivable use cases and barricades for watery zinc batteries, specifically the way that they could deal with outrageous temperatures.
“The watery electrolyte of the Zn battery will be frozen at low temperatures, so we are investigating how to address the temperature impact on battery execution,” Liu said.
More information: Yufang Cao et al, Fast Zn2+ mobility enabled by sucrose modified Zn2+ solvation structure for dendrite-free aqueous zinc battery, Nano Research (2022). DOI: 10.1007/s12274-022-4726-3