A common, economic, and easy method of administering drugs is orally, by swallowing a pill or capsule. But oral administration is the most complex way for the human body to absorb an active pharmaceutical ingredient, because the bioavailability of the drug in the gastrointestinal tract depends on the medication’s ingredients and the stomach’s dynamic physiological environment.
In Physics of Fluids, by AIP Publishing, researchers from Johns Hopkins University and Johns Hopkins School of Medicine employ a biomimetic in-silico simulator based on the realistic anatomy and morphology of the stomach — a “StomachSim” — to investigate and quantify the effect of body posture and stomach motility on drug bioavailability.
“Oral administration is surprisingly complex despite being the most common choice for drug administration,” said co-author Rajat Mittal. “When the pill reaches the stomach, the motion of the stomach walls and the flow of contents inside determine the rate at which it dissolves. The properties of the pill and the stomach contents also play a major role.
“However, current experimental or clinical procedures for assessing the dissolution of oral drugs are limited in their ability to study this, which makes it a challenge to understand how the dissolution is affected in different stomach disorders, such as gastroparesis, which slows down the emptying of the stomach.”
Oral administration is surprisingly complex despite being the most common choice for drug administration. When the pill reaches the stomach, the motion of the stomach walls and the flow of contents inside determine the rate at which it dissolves. The properties of the pill and the stomach contents also play a major role.
Rajat Mitta
Most marketed drugs are administered orally, despite the complex process of oral absorption that is difficult to predict. Oral bioavailability is dependent on the interplay between many processes that are dependent on both compound and physiological properties. Because of this complexity, computational oral physiologically-based pharmacokinetic (PBPK) models have emerged as a tool to integrate these factors in an attempt to mechanistically capture the process of oral absorption.
After oral administration of a drug, absorption into the bloodstream occurs in the stomach and intestine, which usually takes about one to six hours. The rate of absorption depends on factors such as the presence of food in the intestine, the particle size of the drug preparation, and the acidity of intestinal contents.
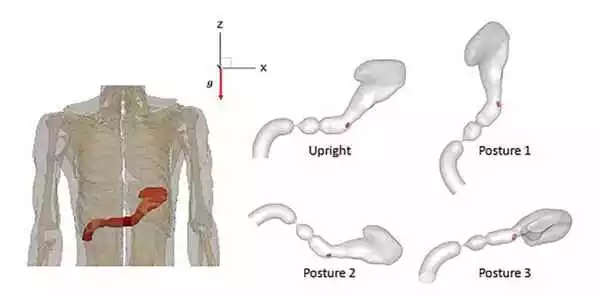
Stomach contents, motility, and gastric fluid dynamics all play a role in a drug’s bioavailability, and stomach contractions can induce pressure and generate complex pill trajectories. This results in varying rates of pill dissolution and nonuniform emptying of the drug into the duodenum and, sometimes, gastric dumping in the case of modified-release dosage.
Together, these issues pose several challenges for the design of drug delivery.
“In this work, we demonstrate a novel computer simulation platform that offers the potential for overcoming these limitations,” said Mittal. “Our models can generate biorelevant data on drug dissolution that can provide useful and unique insights into the complex physiological processes behind the oral administration of pills.”
The modeling appears to be the first of its kind to couple gastric biomechanics with pill movement and drug dissolution to quantify an active pharmaceutical ingredient passing through the pylorus into the duodenum. The model enabled the researchers to calculate and compare the emptying rate and the release of a dissolved active pharmaceutical ingredient into the duodenum for a variety of physiological situations.