Researchers propose a new line of attack for treatment-resistant cancers. The immune system has evolved to protect the body from a wide range of potential threats. Among these are bacterial diseases such as plague, cholera, diphtheria, and Lyme disease, as well as viral infections such as influenza, Ebola virus, and SARS CoV-2.
Despite the impressive power of the immune system’s complex defense network, one type of threat is particularly difficult to combat. This occurs when the body’s own native cells go rogue, resulting in cancer. Although the immune system frequently attempts to rid the body of malignant cells, its efforts are frequently thwarted as the disease progresses unchecked.
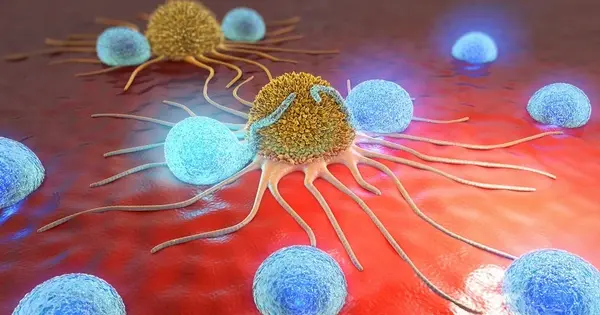
A cancer cell (center) is surrounded by immune T-cells augmented with an oncolytic (cancer-fighting) virus in the illustration. A new study describes how a combination of immunotherapy and virotherapy using the myxoma virus gives patients with treatment-resistant cancers new hope. Grant McFadden, Masmudur Rahman, and their colleagues propose a new line of attack for treatment-resistant cancers in new research published in the journal Cancer Cell.
The strategy combines two methods that have shown significant success against certain cancers. The study describes how oncolytic virotherapy, a technique that uses cancer-fighting viruses, can work in tandem with existing immunotherapy techniques to improve the immune system’s ability to target and destroy cancer cells.
We’re on the verge of learning more about the myxoma virus and oncolytic virotherapy. These findings also pave the way for testing cancer-killing viruses in combination with other cell-based cancer immunotherapies that can be used in cancer patients.
Masmudur Rahman
Oncolytic viruses are an exciting new cancer treatment option. These viruses have a remarkable ability to hunt and kill cancer cells while leaving healthy cells alone, as well as to improve the immune system’s ability to recognize and kill cancer cells.
One such virus, known as myxoma, is the focus of the current research and an area of expertise for the research group. The study shows that the use of T-cells infected with myxoma virus can induce a form of cancer cell death not previously observed.
Known as autosis, this form of cell destruction may be particularly useful against solid tumors that have proven treatment-resistant to various forms of cancer therapy, including immunotherapy alone.
“This work affirms the enormous potential of combining virotherapy with cell therapy to treat currently intractable cancers,” McFadden says. McFadden directs the Biodesign Center for Immunotherapy, Vaccines and Virotherapy at Arizona State University.
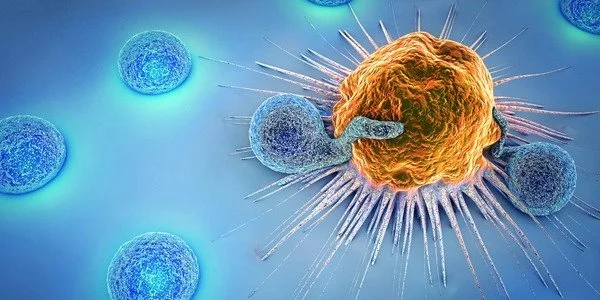
Internal sentries
The immune system is composed of a range of specialized cells designed to patrol the body and respond to threats. The system is involved in a ceaseless arms race against pathogens, which evolve sophisticated techniques to attempt to outwit immune defenses, propagate in the body and cause disease. Cancer presents a unique challenge to the immune system as tumor cells often lack the identifying cell features that allow the immune system to attack them by distinguishing self from non-self.
Cancer cells can further evade immune efforts to hunt and destroy them by employing a variety of evasive strategies. Researchers hope to aid the immune system in overcoming cancer’s notorious disguise tactics by developing new experimental techniques in the category of adoptive cell therapy, or ACT.
Such procedures frequently entail removing a group of cancer-fighting white blood cells known as T-cells, modifying their seek-and-destroy abilities, and reinjecting them into patients. The new study describes two types of ACT immunotherapy: CAR T-cell therapy (CART) and T Cell Receptor Engineering (TCR). In each case, the basic idea is the same: treat cancer with activated T lymphocytes extracted from the patient.
New method delivers one-two punch to tumor cells
The development of these therapies has been nothing short of revolutionary, and some cancer patients facing grim prospects have made remarkable recoveries following the use of immunotherapy. But techniques like CART and TCR nevertheless have their limitations and are often ineffective against advanced solid tumors. In such cases, cancer cells often manage to evade destruction by T-cells by downregulating or losing the surface antigens or MHC proteins that T-cells use to identify them.
The new study highlights the ability of immunotherapy when it is coupled with virotherapy to break through the wall of cancer resistance, specifically using myxoma-equipped T-cells. The myxoma can target and kill cancer cells directly but more usefully can induce an unusual form of T-cell directed cell death known as autosis. This form of cell death augments two other forms of programmed cancer cell death induced by T-cells, known as apoptosis and pyroptosis.
During myxoma-mediated autosis, cancerous cells in the vicinity of those targeted by the therapy are also destroyed in a process known as bystander killing. This effect can considerably enhance the dual therapy’s aggressive eradication of cancer cells, even in notoriously hard-to-treat solid tumors.
A combined myxoma-immunotherapy approach has the potential to convert “cold tumors” that are invisible to the immune system into “hot tumors” that immune cells can identify and destroy, allowing CAR T-cells or TCR cells to enter the tumor environment, proliferate, and activate.
“We’re on the verge of learning more about the myxoma virus and oncolytic virotherapy,” Rahman says. “These findings also pave the way for testing cancer-killing viruses in combination with other cell-based cancer immunotherapies that can be used in cancer patients.”
The ability to radically reengineer oncolytic viruses like myxoma to target a variety of resistant cancers opens up a new avenue for treating this deadly disease.