At the point when natural toxins like colors, agrarian synthetic substances, and drugs enter streams from one side of the planet to the other, they can hurt the climate and human wellbeing, and eliminating them can be unimaginably troublesome.
In a process known as mineralization, photocatalysts—substances that take in light energy and use it to speed up a chemical reaction—can turn organic pollutants into water, carbon dioxide, and other harmless molecules. However, there is a catch: the majority of photocatalysts are impractical and costly to use on a large scale because they require UV light to function.
To tackle that issue, specialists have their sights set on finding a photocatalyst that can outfit significantly more of the sun-oriented range. ” Magnus Rnning, a catalysis professor at the Department of Chemical Engineering at NTNU, says, “If you can use solar light, it’s cheaper and much more available than UV light.”
“It’s a fairly typical organic contaminant, but it’s also a fairly complex molecule. It should work well on other organics if it works on methylene blue.”
Magnus Rønning, a professor in catalysis at the Department of Chemical Engineering, NTNU.
Gold nanoparticles key
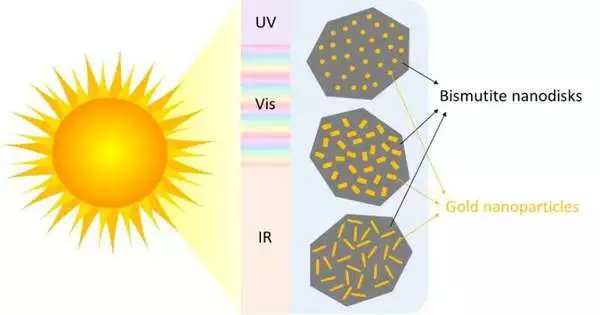
Adding gold nanoparticles to the surface of nano-sized disks made of the mineral bismuth makes them more sensitive to the visible spectrum of the sun, making them a promising photocatalyst. Notwithstanding, while there are multiple ways of saving those gold nanoparticles on a surface, most techniques give you restricted control over the size and state of the particles you end up with.
According to Rnning, “most of the time you will get a distribution of sizes and shapes [that] you really cannot control, so you have a mix of rods, spheres, and cubes.”
Presently, Rnning and partners at NTNU have figured out how to make gold nanoparticles with a uniform size and shape on the outer layer of the bismutite nanodisks. The results of their study, which were recently published in the journal Photochemical and Photobiological Sciences, make it possible to investigate how the catalyst’s performance is affected by the size and shape of the nanoparticles, thereby increasing the catalyst’s capacity for capturing light.
Testing various nanoparticle shapes
The researchers grew gold nanoparticles in various shapes—rods, etched rods with roughened surfaces, and spheres—by adjusting the concentration and pH of the solution they were growing in. They used small gold seeds as nucleation sites.
Before being deposited on the bismutite nanodisks, the surface of these nanoparticles has a surfactant layer that reduces the likelihood of clumping.
According to Rnning, “with this, we can maintain reasonably good control over the size and shape of these particles.” Ph.D. candidate Jibin Antony prepared and characterized the samples in NTNU’s NanoLab, while the Catalysis Group in the Department of Chemical Engineering performed the catalytic reactions themselves.
The researchers tested the photocatalyst’s ability to break down methylene blue, an organic pollutant. As well as being similar to a far-reaching natural impurity by its own doing, methylene blue is a helpful experiment for how well a photocatalyst will deal with different poisons.
According to Rnning, “It’s quite representative as an organic contaminant, but it’s also a relatively complex molecule.” It should work well on other organics if it works on methylene blue.”
The other benefit of utilizing methylene blue is that its disintegration is as of now surely known, permitting the analysts to test not exactly how much methylene blue is left toward the end of the cycle but what it’s been switched over completely to.
In their work on gold nanoparticles, Rnning and colleagues didn’t look into that, but in a related paper, they found that adding silica to bismutite nanodisks changed the degradation products of methylene blue. Rnning says, “In the end, you want full mineralization, not just conversion into something that is just as dangerous or undesirable as methylene blue.”
Bars are better compared to circles.
Rnning and his associates found that the photocatalyst with bar-formed gold nanoparticles performed 14% better than the one with circles. Yet, there is still opportunity to get better. ” “You still have some of the contaminant left over even after three hours of reaction,” says Rnning. Therefore, it is effective. However, we still require something that functions better.
Commercial air purification and wastewater treatment systems already make use of some photocatalysts. Hydrogen splitting, which uses only water and sunlight to produce inexpensive hydrogen fuel, holds promise for the technology as well. However, in order for that to be possible, researchers will need to devise a strategy that will significantly enhance the catalysts’ current effectiveness.
The key improvement required for better photocatalysts lies in the number of photons that are really used to drive the response. ” According to Rnning, “it’s probably 1% in good cases.” It will be much closer to practical applications if we can get this up to, say, 10%.”
While changing the size and state of gold nanoparticles is probably not going to achieve such an immense expansion in proficiency, it’s a beginning. “We want to work on the impetus for this to be financially suitable,” says Rnning. “That is a first step, and this is it.”
More information: Jibin Antony et al, Optimizing the shape anisotropy of gold nanoparticles for enhanced light harvesting and photocatalytic applications, Photochemical & Photobiological Sciences (2022). DOI: 10.1007/s43630-022-00351-8