Late pressure to improve antibody adequacy has resulted in numerous new discoveries in immunology, revealing various standards with previously unknown beneficial potential.One developing part of the examination is centered around tissue-occupant memory lymphocytes (TRM cells), a sort of safe cell that gives durable security against microbes going after unambiguous organs and tissues.
In another review distributed December 28, 2022, in Resistance, researchers at the College of California San Diego Institute of Medication uncovered a formerly neglected intricacy of TRM cell science in the stomach, which might usher in another age of accuracy therapeutics against contamination, malignant growth, and auto-safe illness.
Subsequent to encountering a disease, the safe framework abandons memory lymphocytes, which keep a durable sub-atomic memory of the microbe and are prepared to sound the alarm in the event that it at any point returns. While some memory lymphocytes are intended to flow through the circulatory system and give the entire body security, others live in unambiguous organs and are specific to battling the microbes that focus on that piece of the body. These TRM cells can provide deep-rooted resistance at the objective tissue level, but if overactivated, they can also contribute to immune system illnesses.
“TRM cells are the people on call, right at the forefronts of disease,” said senior creator and UC San Diego Institute of Medicine professor John T. Chang, MD.”While the majority of our antibodies are designed to provide fundamental resistance, we may be able to achieve far better security by focusing on helping the tissue-explicit cells that encounter the microbe first.”
For instance, a respiratory infection might be best battled by reinforcing TRM cells in the nose and lungs, and a pathogenic stomach organism might be best treated by upgrading TRM cells in the digestion tracts. Hence the objective is to foster therapeutics that could help the development and upkeep of TRM cells or, on account of immune system illness, eliminate the safe cells by upsetting these equivalent pathways.
The issue is that researchers actually have a long way to go in figuring out what helps TRM cells structure and get by, and these standards might be very divergent in each tissue type.
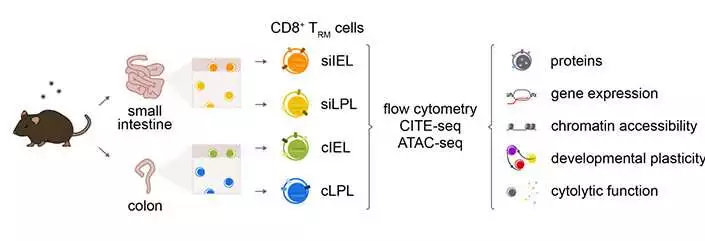
Researchers in the Chang lab at UC San Diego examined TRM cells from the lamina propria (LPL) and epithelial (IEL) compartments of the mouse small digestive tract and colon, uncovering huge transcriptional, epigenetic, and useful heterogeneity across the four cell populations. Wellbeing Sciences at UC San Diego
To investigate this, the scientists played out a progression of tests to portray TRM cells in mice from four unique compartments of the stomach: two organs (the small digestive tract and the colon) and two different tissue layers in each (the intraepithelial and lamina propria layers).
The tests uncovered that TRM cells in each tissue type showed particular examples of cytokine and granzyme articulation, along with significant transcriptional, epigenetic, and useful heterogeneity. As such, similar-looking safe cells in each piece of the stomach had all the earmarks of being totally different in their atomic cosmetics, capabilities, and the compound signals they rely upon.
Supporting this further, every population of cells likewise showed differential reliance on Eomesodermin (Eomes), a transcriptional factor known to influence TRM cell improvement. Eomes was traditionally thought to curb TRM cells in view of past information gathered from the skin, liver, and kidney, yet the new tests uncovered the inverse was valid in the small digestive tract. There, Eomes ended up being shockingly significant in the endurance of TRM cells. Nonetheless, this was not the situation in the colon, which featured high levels of explicitness even inside the stomach.
Future exploration will keep on characterizing the standards of TRM cell arrangement and upkeep in different tissues and investigate what drives their explicitness. For instance, the creators propose that distinctions in the microbiome of the small digestive tract and the colon might add to the novel necessities of their TRM cells, so controlling the microbiome might be one more way to deal with managing safe cells in the stomach.
“Later on, we need to ponder antibodies and different therapeutics that are customized to the particular necessities of every organ,” said Chang. “By understanding what each tissue type needs to help the development and upkeep of TRM cells, we can give the most effective safe guards against illness.”
More information: Yun Hsuan Lin et al, Small intestine and colon tissue-resident memory CD8+ T cells exhibit molecular heterogeneity and differential dependence on Eomes, Immunity (2022). DOI: 10.1016/j.immuni.2022.12.007
Journal information: Immunity