Electrochemical gadgets, for example, like energy units, are becoming key for new power-age advances since they can effectively create sustainable power. Clay proton guides can be utilized in numerous applications, including proton-fired energy units (PCFCs), hydrogen siphons, sensors, and division films. Specifically, PCFCs in view of clay proton guides are promising on the grounds that they can work at lower temperatures compared to regular strong oxide energy units (SOFCs), because of the greater conductivity of protons at low temperatures.
In any case, regular clay proton guides deal with one issue: to show sufficient proton conductivity, they need to have oxygen opening the water fuse. As a rule, the openings are made through compound replacement, which is often a troublesome cycle.
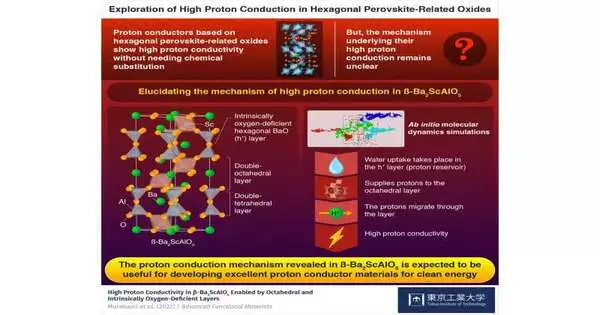
Presently, all things considered, a gathering of scientists led by Teacher Masatomo Yashima of Tokyo Tech’s Branch of Science has investigated the proton-leading hexagonal perovskite-related oxides. The gem design of these oxides contains layers that are naturally oxygen-lacking, which empowers high proton conductivity without compound replacement. In any case, their conduction system stays hazy.
“It is made up of double-octahedral layers that are separated by double-tetrahedral layers. The latter have hexagonal BaO (h’) layers that are oxygen deficient by nature. Various methods have been used to investigate their functions in proton conduction.”
Professor Masatomo Yashima of Tokyo Tech’s Department of Chemistry
To reveal insight into this, the examination bunch driven by Teacher Yashima as of late dissected and analyzed three sorts of oxides: -Ba2ScAlO5, -Ba2Sc0.83Al1.17O5, and BaAl2O4. The oxide-lacking layers of this multitude of three oxides have different stacking designs. The researchers discovered that while -Ba2ScAlO5 had a high proton conductivity, the closely related -Ba2Sc0.83Al1.17O5 and BaAl2O4 had much lower conductivities.
The outcomes of the gathering were disseminated in cutting-edge useful materials.
Prof. Yashima explains the gem design of -Ba2ScAlO5 briefly: “It consists of twofold octahedral layers isolated by twofold tetrahedral layers.”The last option has hexagonal BaO (h’) layers that are naturally oxygen-deficient. “Their jobs in proton conduction have been investigated through different techniques.”
To begin, the researchers discovered that the particle conductivity of -Ba2ScAlO5 was many-fold (multiple times) higher in wet air than in dry air.This was because the material retained water from the wet air, prompting higher proton concentrations and conductivity. Over 300 °C, the proton conductivity was measured to be as high as 103 S cm1—a value comparable to that of regular, artificially subbed guides.
Bond valence-based energy and thickness estimations uncovered that this water take-up happens in the “h” layers of the oxide. Furthermore, stomach muscle atomic element recreations demonstrated that these layers function as repositories, supplying protons that move through long reach dispersion in the twofold octahedral layers.This peculiarity accounts for -Ba2ScAlO5’s high proton conductivity.
Conversely, BaAl2O4 showed a lot of lower conduction because of less water take-up, low proton versatility, and the shortfall of octahedral layers. These perceptions further approve the huge jobs of both octahedral and oxygen-lacking layers in proton conduction.
“The review is an extraordinary instance of handling complex exploration issues through cooperation and features ANSTO capacities and skills in neutron dispersing and logical figuring.” “The Echidna diffractometer at the OPAL reactor was utilized to clarify gem structure, and sub-atomic element recreations likewise performed at ANSTO shed light on the proton conductivity system,” said Prof. Max Avdeev of ANSTO.
Prof. Yashima examines the future capability of the cooperation: “Our outcomes offer a system for planning unrivaled hexagonal perovskite-related oxides with octahedral layers and naturally oxygen-lacking layers.” “Joining these layers with various jobs can create unrivaled proton guides for sustainable power creation and capacity gadgets.”
More information: Taito Murakami et al, High Proton Conductivity in β‐Ba 2 ScAlO 5 Enabled by Octahedral and Intrinsically Oxygen‐Deficient Layers, Advanced Functional Materials (2022). DOI: 10.1002/adfm.202206777