Cancer cells in the most well-known pancreatic disease share supplements to live and develop. Another disclosure by College of California, Irvine scholars and partners during a four-year examination could assist with improving therapies for pancreatic ductal adenocarcinoma, which represents nearly 90% of pancreatic disease cases. The researchers’ paper shows up in Nature Disease. While pancreatic disease is uncommon, it is one of the leading causes of cancer death in the United States.
One snag in treating pancreatic ductal adenocarcinoma, known as PDA, is that it by and large doesn’t show early side effects. One more obstacle is the intricacy of its thick and sinewy cancers. Thus, they don’t have completely working veins in the growth. On one front, this makes it hard to convey viable chemotherapy. However, it also implies that the cancers have promoted a different type of digestion.
“Without veins, PDA cells aren’t getting the typical supplements they require, so they’ve devised alternate methods of feeding themselves and developing,” said Christopher Halbrook, an assistant professor of atomic science and natural chemistry and the study’s lead and co-creator.Understanding this cycle is fundamental for concocting therapies focusing on the disease’s digestion.
“Without blood arteries, PDA cells don’t acquire the typical nutrition they require, so they’ve evolved different ways to nourish themselves and develop,”
Christopher Halbrook, assistant professor of molecular biology & biochemistry, and lead
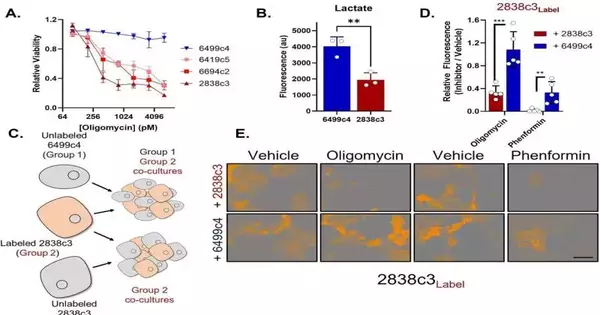
The researchers discovered two distinct types of PDA disease cells within growths, each with its own set of metabolic cycles.”This differential programming permits them to trade supplements, so each gets what it needs,” Halbrook said.
The group observed that one class of cells is powerless against mitochondrial harm, frequently utilized as disease treatment, yet it gets by with the assistance of the aminocorrosive asparagine it gets from the other cell class. The last option has a lot to share since they overproduce asparagine because of continuous pressure.
“Once we realized this, we discovered we could use the protein asparaginase to restore the cancers’ vulnerability to mitochondrial damage,” Halbrook explained.
The group’s discoveries yield significant information for further developing therapy for pancreatic malignant growth and possibly for different illnesses. “With cancers, we can’t expect there to be a homogeneous population to target,” said Halbrook. “Assuming we will have digestion-based treatments, it will be vital to focus on various cell ways of behaving.”
The researchers approved the metabolically beneficial interaction model utilizing cloned human and mouse PDA cancer cells and single-cell RNA sequencing. Then, utilizing pre-clinical models, they made another significant finding.
“At the point when we treated cancers with Phenformin, a strong mitochondrial poison, along with asparaginase, it halted growth development,” said Halbrook. He added that there is something else to master, including recognizing the reason for the continuous pressure in the help cells that drives the beneficial interaction.
More information: Christopher J. Halbrook et al, Differential integrated stress response and asparagine production drive symbiosis and therapy resistance of pancreatic adenocarcinoma cells, Nature Cancer (2022). DOI: 10.1038/s43018-022-00463-1
Journal information: Nature Cancer