Some of the time the best things in life happen by coincidence, when we end up being perfectly located. Presently, scientists from Japan have figured out how to guarantee that new drugs are conveyed to the perfect locations in the body at the ideal timepoint in sickness movement, so they make the best difference.
In a review distributed as of late in the Journal of Nanobiotechnology, scientists driven by the Tokyo Clinical and Dental College (TMDU) have uncovered that a clever conveyance framework conveys treatment to where it is needed most in a mouse model of Alzheimer’s disease (Promotion).
Promotion is a typical neurodegenerative illness that causes dementia. It is portrayed by the gathering of a protein called amyloid (A) in the mind, and various different harmful types of A have been recognized that debilitate cerebrum capability, notably A oligomers (AOs).
“Many clinical trials have sought to employ an anti-A antibody to treat Alzheimer’s disease, but the results have been disappointing. One possible explanation for this is that the BBB blocks most full-length antibodies from accessing the brain.”
Lead author of the study Akiko Amano.
“Various clinical preliminary studies have endeavored to utilize an enemy of A neutralizer to treat promotion, yet the outcomes have been unacceptable,” says the lead creator of the review, Akiko Amano. “One possible clarification for this is that the blood-cerebrum obstruction (BBB) keeps most full-length antibodies from entering the mind.”
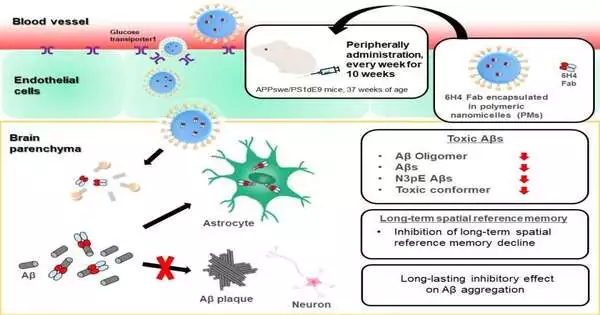
To address this test, the scientists recently created glucosylated (sugar-connected) polymeric nanomicelles (PMs), which are minuscule, empty balls that could effectively cross the BBB through transcytosis in mouse cerebrum slender endothelial cells; this cycle was interceded by glucose-carrier 1 and actuated by an expansion in blood glucose levels after the mice experienced fasting conditions.
In this review, Takanori Yokota and partners filled PMs with pieces of an enemy of the AO immunizer, infused them into a mouse model of promotion, and surveyed the impacts on the mind and on conduct.
“The outcomes were exceptionally clear,” says senior creator Nobuo Sanjo. “The organization of anti-AO neutralizer sections through PMs fundamentally decreased the measures of different harmful A species. Also, the A plaques that were framed were more modest and less thick than those seen in untreated mice.”
Then, the specialists dissected the way the mice behaved and found that the mice treated with the immunizer-part-filled PMs would do better in terms of learning and spatial memory than untreated mice. “Our discoveries propose that conveying adequate degrees of antibodies to the mind using PMs can lessen harmful A species and slow Promotion movement in mice,” says Amano.
Considering that the failure of anti-A antibodies to work on mental capability in human clinical preliminaries was reasonable on account of a deficient stockpile of the antibodies in the cerebrum, PM-embodied counteracting agent sections could address a viable method for forestalling the Promotion movement. Furthermore, a new contender for promotion treatment that debases poisonous As and decreases their harmful impacts could likewise be conveyed to the mind utilizing a similar PM-based framework.
More information: Akiko Amano et al, Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain, Journal of Nanobiotechnology (2023). DOI: 10.1186/s12951-023-01772-y