A group of scientists from Helmholtz Munich, the German Center for Diabetes Research (DZD), and Novo Nordisk have fostered another chemical mix for the future treatment of type 2 diabetes. The researchers have joined the glucose-lessening impacts of the medications tesaglitazar and GLP-1 (Glucagon-like peptide-1) in a new and profoundly viable medication. That’s what the benefit is. By joining tesaglitazar with GLP-1, the tesaglitazar just enters tissue that contains GLP-1 receptors. This lessens the unfriendly impacts of tesaglitazar while increasing the consequences for sugar digestion. The new medication has previously been effectively tried in creature studies. The discoveries were published in Nature Metabolism.
The medication tesaglitazar further increases glucose and fat digestion in patients with type 2 diabetes. It follows up on two receptors inside the cell core to increase insulin awareness. This was demonstrated in stage 3 clinical preliminaries. Nonetheless, tesaglitazar also caused undesirable impacts, for example, indications of kidney harm. The scientists used a trick to get the medication to work properly: they biochemically linked tesaglitazar with the gastrointestinal chemical GLP-1, which has long been used to treat type 2 diabetes.This permits the joined medication to just follow up on cells and tissue that contain GLP-1 receptors.
“This stunt empowered us to join the glucose-lessening impacts of GLP-1 and tesaglitazar into a solitary profoundly viable particle, while keeping tesaglitazar away from tissues that it could harm,” makes sense of PD Dr. Timo Müller, the related creator, head of the Institute of Diabetes and Obesity, and researcher at DZD.
The new medication further develops glucose resistance and sugar digestion.
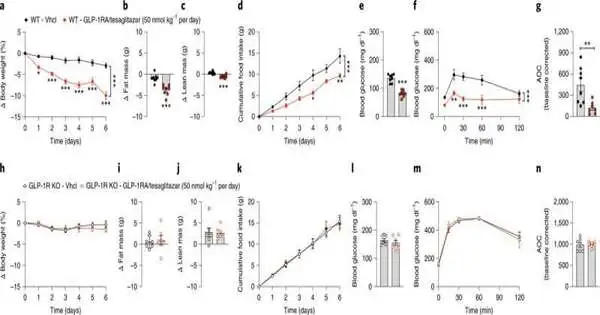
“The sugar metabolism of obese and diabetic male mice improved to a significantly larger extent compared with treatment utilizing only the GLP-1 hormone or tesaglitazar alone—and with no detrimental adverse effects to the liver or kidney,”
Prof. Kerstin Stemmer, one of the lead authors of the study.
The new medication has previously been effectively tried in creature studies: “The sugar digestion of fat and diabetic male mice improved to a far more prominent degree compared to treatment utilizing just the GLP-1 chemical or tesaglitazar alone—and with no harmful unfriendly impacts to the liver or kidney,” says Prof. Kerstin Stemmer, one of the lead creators of the review.
The substance was especially viable in expanding glucose resilience levels. Just negligible dosages of the new medication were expected to accomplish feasible improvement in glucose digestion. “This medication has incredible potential for the intensive therapy of raised glucose levels related to type 2 diabetes,” says Aaron Novikoff, another lead creator of the review.
The analysts currently need to examine whether this medication can also possibly treat people with type 2 diabetes and whether the adequacy of this new mix treatment can be upgraded further by utilizing biochemical changes.
The group, including Prof. Dr. Matthias Tschöp (logical head of Helmholtz Munich), PD Dr. Timo Müller, Richard DiMarchi, Ph.D. (Indiana University), and Dr. Brian Finan (Novo Nordisk), has been working for a long time on new medication contenders for the treatment of type 2 diabetes and weight. All the while, poly-agonists copy the impacts of different chemicals. Clinical preliminaries have shown that some poly-agonists are especially encouraging in the treatment of weight and type 2 diabetes and are now in stage 2 and stage 3 preliminaries. This year, in the U.S., the main double agonist drug for the treatment of type 2 diabetes was endorsed. The medication copies the impacts of GLP-1 and GIP (glucose-subordinate insulinotropic polypeptide) receptor agonists that copy the impacts of the digestive chemicals GLP-1 and GIP.
More information: Carmelo Quarta et al, GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice, Nature Metabolism (2022). DOI: 10.1038/s42255-022-00617-6
Journal information: Nature Metabolism