Phenanthridines are heterocyclic compounds in which two benzene rings with six members fuse to form a nitrogen-containing six-membered ring. They can be found in a lot of organic compounds that are naturally occurring and have anticancer and antitumor properties. There is a lot of interest in making phenanthridine derivatives in the lab because they could be used in medicine.
Imidoyl radical intermediates, which cyclize to produce phenanthridine, are produced by radical isonitrile insertion, a promising synthesis strategy. The precise mechanism of isonitrile insertion, on the other hand, is poorly understood.
The use of aryl-substituted difluoromethylborates in the production of difluoromethylated phenanthridines has recently been the subject of an investigation by a group of researchers led by Associate Professor Shigekazu Ito from the Tokyo Institute of Technology (Tokyo Tech). Their research, which was published in The Journal of Organic Chemistry, explains how isonitrile radical addition reacts with aryl-substituted difluoromethylborates to produce pharmaceutically useful fluorinated phenanthridines.
“Given the importance of difluoromethylated phenanthridines in drug discovery, it is desirable to develop novel and complementary synthetic methods for producing 6-(difluoromethyl)phenanthridines, particularly via radical isonitrile insertion.”
Associate Professor Shigekazu Ito from Tokyo Institute of Technology (Tokyo Tech),
Dr. Ito says, “It is desirable to develop novel and complementary synthetic methods for producing 6-(difluoromethyl)phenanthridines, especially via radical isonitrile insertion,” given the significance of difluoromethylated phenanthridines in drug discovery.
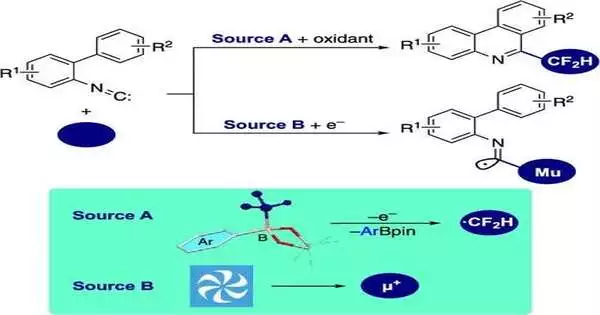
By first oxidizing aryl-substituted difluoromethylborates, the researchers produced a highly reactive difluoromethyl radical (•CF2H) for the production of 6-(difluoromethyl)phenanthridines. Within the isonitrile group, the isonitrile insertion and cyclization processes were initiated by this radical.
Silver oxide (Ag2O) and potassium peroxodisulfate (K2S2O8) were found to be ideal initiators for radical isonitrile insertion in 2-isocyano-1,1′-biphenyls after screening various oxidizing conditions. K2S2O8 oxidized Ag2O, which in turn oxidized the aryl-substituted difluoromethylborates and produced the •CF2H radical, as they observed. It binds to the isonitrile group, creating the imidoyl radical. This radical then undergoes intramolecular cyclization, resulting in the synthesis of 6-(difluoromethyl)phenanthridine.
To get the most 6-(difluoromethyl)phenanthridine out of aryl-substituted difluoromethylborates, the researchers looked into a variety of aryl groups. P-diethylamino-phenyl-substituted borate was the most stable of the aryl groups that were tested, and it produced the corresponding phenanthridine in a respectable 53% yield.
“Transverse-field muon spin rotation” was used by the researchers to confirm the reaction mechanism and the presence of the short-lived imidoyl radical. The isonitrile group was targeted by a beam of positive muons, subatomic particles nine times lighter than protons, and their spin changes were carefully observed.
Muoniums, which are muons that accompany electrons, are preferentially added to the carbon atom of the isonitrile unit, resulting in a cyclization intermediate. The elusive imidoyl radical’s existence was solidly established by this observation.
For the purpose of facilitating the production of difluoromethylated phenanthridines, the team hopes to investigate various methods for producing the difluoromethyl radical in the future. Dr. Ito says that, in addition to chemical oxidation, photocatalytic and electrochemical methods might be used to synthetically generate the difluoromethyl radical from difluoromethylborates.
In conclusion, this research offers a novel and extremely promising method for the synthesis of 6-(difluoromethyl)phenanthridines, which has enormous potential for drug development.
More information: Kakeru Konagaya et al, Difluoromethylborates and Muonium for the Study of Isonitrile Insertion Affording Phenanthridines via Imidoyl Radicals, The Journal of Organic Chemistry (2023). DOI: 10.1021/acs.joc.3c00056