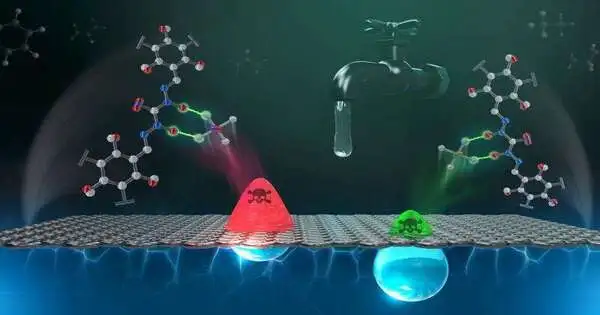
Specialists at the Department of Energy’s Oak Ridge National Laboratory are handling a worldwide water challenge with an exceptional material intended to target not one, but two poisonous, weighty metal contaminations for synchronous evacuation.
In genuine circumstances where water assets contain numerous artificially comparable components, ORNL’s Santa Jansone-Popova of the Chemical Sciences Division, and Ping Li, presently at Elementis Global, have found an adsorbent with high selectivity for chromium and arsenic.
Results distributed in Small show the new material catches chromium and arsenic in a reasonable 2-to-1 proportion. The crucial development makes collaboration between chromium and arsenic possible, with the goal that the more chromium the material snatches, the more arsenic it can likewise eliminate.
“It is unusual for an adsorbent to capture two pollutants at the same time, and to work quickly and efficiently in realistic scenarios to address a wide range of water conditions around the world.”
Santa Jansone-Popova of the Chemical Sciences Division
“It is uncommon for an adsorbent to catch two contaminations all at once, and to work rapidly and proficiently in sensible situations to address the wide scope of water conditions around the world,” Jansone-Popova said.
Chromium and arsenic are two of the most risky toxins found in drinking water around the world. Both are harmful and can cause unfriendly well-being impacts, including malignant growth. Indeed, even low levels pose significant risks to living organic entities because portions bioaccumulate or develop with every openness and can gradually accumulate to toxic levels.These components happen normally, but their presence in the climate has expanded with industry and urbanization as a result of wide mining, creation, and assembling. Discharges influence air, soil, and water, but drinking water is the most widely recognized course of openness.
In water, these metals break up into chromate and arsenate oxoanions, or salts, that are synthetically like gainful minerals that are normally present in water, including phosphate, sulfate, nitrate, and bicarbonate. Chromate and arsenate are exceptionally versatile in water and can have extensive effects. They don’t corrupt and are extremely durable in the climate without mediation. Designated approaches are expected to isolate these metals from innocuous mineral salts that are fundamental to the environment.
It is important for an ORNL group to have some expertise in the investigation of adsorbents, materials that are intended to target explicit components and tie them to a surface. Adsorbents have expansive applications in assisting in recuperating valuable metals or eliminating poisons from the environment.
“They are one of the most encouraging water treatment choices since they are reasonable, handily conveyed, and can work rapidly to channel water supplies, but they should be custom fitted for down to earth use in cleanup situations,” Jansone-Popova said. “The test is to plan materials that can successfully segregate and follow measures of hurtful components that are basically the same as the mass substance species tracked down in water.”
In an adsorbent plan, selectivity is critical. Since a material’s surface offers restricted land, the objective is to get just the designated components and catch as much as could be expected before the adsorbent tops off and should be supplanted or reused. Inadequately particular materials miss the mark on accuracy to single out focus in blended conditions like water, where comparable components go after space.
Jansone-Popova recently drove the development of an adsorbent with high selectivity for chromate that works quickly and within the sight of contending species to sterilize water. A review published in Environmental Science and Technology showed the original material diminished chromate fixations 100-overlap in no less than one moment (1 section for every million to 10 sections for each billion) and accomplished a level a significant degree below reasonable cutoff points set by the U.S. Natural Protection Agency.
The collaboration with Ping Li expands on the way to deal with fostering a structure for catching both chromate and arsenate with one material.
“Our beginning material is profoundly compelling at catching chromium in its most harmful structure, hexavalent chromium, yet the methodology was not intended to be particular for arsenic,” Li said. “As this response occurs, in any case, the material changes, making a stage for new sciences.”
Specialists changed the first design to reduce chromium-6 into a less poisonous state, chromium-3. Chromium-3 additionally has the advantage of giving an anchor highlight tie arsenate. The new design empowers a substance response that structures stable chromate-arsenate groups that are firmly attached to the surface. The outcome successfully traps the poisons for all time since they won’t wash off or confine them from the channel material without deliberate evacuation by synthetic handling.
“We utilized the effective catch of hexavalent chromium to present another engineering that could likewise tie with arsenic,” Li said.
Chromate arsenate, when utilized as an added substance in pressure-treated amble, gave motivation to the construction.
The group has licensed the construction and is working with associates to extend the way to deal with other ecological contamination.
“Basic disclosures like these can assist us with diminishing poisonous toxins in the environment and meet administrative objectives for clean water,” Jansone-Popova said.
More information: Ping Li et al, Bifunctional Ionic Covalent Organic Networks for Enhanced Simultaneous Removal of Chromium(VI) and Arsenic(V) Oxoanions via Synergetic Ion Exchange and Redox Process, Small (2021). DOI: 10.1002/smll.202104703