The building blocks of a design are comprised of burden-bearing components that seldom change in spite of remodels or fixes. They stay in salvageable shape and are steady after some time, yet in the human body, our structural blocks do the polar opposite.
Bones are dynamic: They continually separate and revamp to turn into the most grounded adaptations of themselves.
A transformation in this bone recovery cycle can prompt powerless and delicate bones, and on the off chance that the change is connected with the created measure of collagen — a protein tracked down in bone, connective tissue, skin, and ligament — it prompts fragile bone illness.
Weak bone sickness, otherwise called osteogenesis imperfecta, as of now affects 20,000-50,000 individuals in the US but, at the same time, is the most ordinarily acquired bone illness. It frequently starts in utero, and much of the time, doctors can see breaks in the child’s appendages before they are even conceived. What’s more, these patients are probably going to break different bones during their lifetime. Meenal Mehrotra, M.D., Ph.D., is an associate teacher in the division of medical procedure at the Clinical College of South Carolina, and she explains that there is at present no solution for the illness. She also draws attention to the fact that doctors primarily focus on treating the side effects of weak bones, which can have its own set of complications.
“Bone-resorbing cells known as osteoclasts gnaw away at the damaged area. And then osteoblasts, which are bone-forming cells, come in and replace that piece of the bone.”
Meenal Mehrotra, M.D., Ph.D., is an assistant professor in the department of surgery
She wants to track down a more secure strategy to treat this staggering infection.
As per Mehrotra, solid bone continually encounters and rebuilds its own microfractures. “Bone-resorbing cells known as osteoclasts bite away at the impacted region,” she said. “And afterward, osteoblasts, which are bone-shaping cells, come and reconstruct that piece of the bone.”
At present, meds called bisphosphonates are being utilized to treat fragile bone illnesses, which impact osteoclast levels. By diminishing osteoclasts and in this manner, bone breakdown, there is more bone framed. In any case, Mehrotra says this doesn’t fix the underlying issue, as the bone framework is likewise deficient in nature.
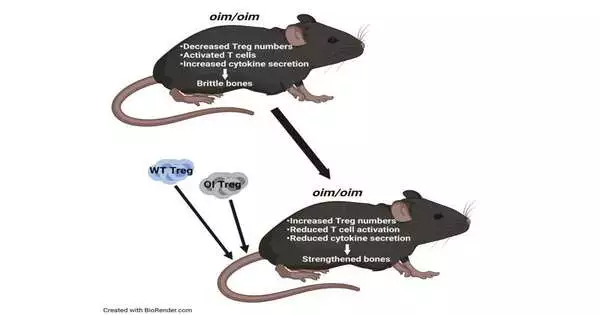
In a new paper in iScience, the group takes a gander at administrative lymphocytes, or Treg cells, as a formerly uninvestigated treatment for weak bone sickness. Treg cells assist in keeping up with equilibrium and homeostasis in the body by keeping the action of the safe cells (lymphocytes) in line. When enacted, white blood cells discharge cytokines, which thus lead to irritation. Yet, when enough Treg cells are available, immune system microorganisms aren’t as exceptionally actuated, and that implies there are fewer cytokines and less irritation.
They observed that the inner climate of mice with this infection is hyperinflamed due to fewer Tregs. Mehrotra speculated that raising these Treg numbers in mice with fragile bone sickness could prompt undiscovered therapy choices.
Through Treg transplantations, specialists demonstrated that raising the quantity of Tregs really does without a doubt improve bone rebuilding, which consequently prompts more grounded bones.
Mehrotra and her group tried Treg cell transplantation with both the impacted mouse’s own immune system microorganisms (autotransplantation) and a benefactor mouse’s lymphocytes (allotransplantation) and looked at the outcomes. While the two strategies brought about more significant levels of Treg cells and more grounded bones, she found that by extricating the subject’s own Treg cells and expanding those levels prior to relocating them back, treatment results may endure longer.
With the two transplantations, Mehrotra saw expanded osteoblast numbers and diminished osteoclast numbers. There was all the more of an equilibrium. “It’s exceptionally energizing,” she said. “We saw better bone design and better bone mechanics. Bones were stiffer.”
However, the most thrilling part for her is that while allotransplantation and autotransplantation both worked, the outcomes with autotransplantation were superior to those seen with allotransplantation. Projecting toward a likely clinical viewpoint, utilizing a patient’s own cells would take into consideration less contributor dismissal and no utilization of immunosuppressants. “It would be simpler for our patients,” she said.
Subsequent to concentrating on hematopoietic immature microorganisms and fragility bone illness in a past report, Mehrotra had the plan to check out Treg cells. Furthermore, when she began researching the topic, she discovered that no one had previously considered Treg cell transplantation as an immunotherapy treatment option for patients with this illness.She is anticipating proceeding with this promising line of exploration later on.
She next needs to take a gander at why a hereditary illness of collagen transformation that apparently has no relationship to insusceptibility conveys an immunological shortage.
Additionally, she needs to see how expanding the Tregs explicitly achieves the positive changes in the bone. “That is the other piece of the puzzle for me,” she said.
Addressing the two inquiries will assist in carrying Treg cell transplantation nearer to facilities for individuals with fragile bone infections.
More information: In-Hong Kang et al, Quantitative increase in T regulatory cells enhances bone remodeling in osteogenesis imperfecta, iScience (2022). DOI: 10.1016/j.isci.2022.104818
Journal information: iScience