Brain tumor research, including gliomas, is an active and complicated field. Targeting individual genes, such as TUG1, to treat brain tumors is an area of interest, but it’s crucial to remember that many gene-based therapies are still in the experimental and preclinical stages. TUG1 is a long non-coding RNA (lncRNA) that has been linked to a variety of cancers, including brain tumors, but it is not the primary cause of these tumors’ genesis or progression.
A new study has discovered a critical link between how cancer cells deal with replication stress and the function of Taurine Upregulated Gene 1 (TUG1). The researchers were able to inhibit brain tumor growth in mice by targeting TUG1 with a medication, suggesting a potential technique to battle aggressive brain cancers such as glioblastomas.
“These findings have the potential to be translated into therapeutic applications, as TUG1 is highly expressed in glioblastoma,” lead researcher Professor Yutaka Suzuki stated. “In this study, we successfully developed a therapeutic drug called TUG1-DDS, which targets TUG1 selectively.” It effectively reduced tumor development and increased survival, especially when combined with the conventional treatment of temozolomide. As a result, it is a potentially promising treatment drug for glioblastoma.”
These findings have the potential to be translated into therapeutic applications, as TUG1 is highly expressed in glioblastoma. In this study, we successfully developed a therapeutic drug called TUG1-DDS, which targets TUG1 selectively.
Professor Yutaka Suzuki
To understand how TUG1 could potentially cure the most serious forms of brain cancer, it is necessary to first understand how cancer hijacks host cell functions in order to establish an environment favorable to cancer cell proliferation. Even essential cell processes, such as replication, are exploited by cancer.
When a cell splits, it copies its DNA, ensuring that both cells have a complete set of genetic information. The double-stranded DNA is unraveled and divided into two single strands, each of which serves as a template for joining with RNA to create two identical copies. An R-loop, a DNA:RNA hybrid structure, aids in the unwinding and stabilization of the DNA.
To improve the conditions for cancer cells, the invasive cells hinder the natural process of DNA replication. The cells induce replication stress (RS), which results in the DNA strands breaking and unpaired single strands of DNA increasing. The result is an instability in the genome that favors tumor growth.
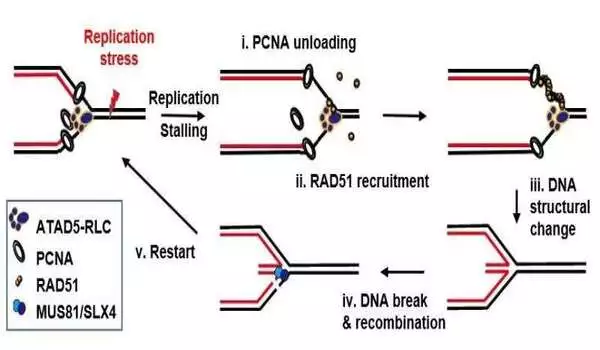
Cancer cells must strike a delicate balance because increased activity has the potential to backfire. Cancer cell death can also be caused by RS and R-loop buildup. Cancer cells use long noncoding RNAs (lncRNAs) to regulate the genome, which allow them to repair their own DNA damage and delete undesirable R-loops.
The role of the lncRNA TUG1 was discovered in a new study headed by Yutaka Kondo and Miho Suzuki at Nagoya University Graduate School of Medicine. TUG1 works with two other proteins, DHX9 and RPA32, to reduce potentially detrimental R-loops. TUG1-RPA-DHX9 interaction is an essential mechanism for regulating R-loops in regions known to be vulnerable to DNA damage and mutations. Nature Communications reported their findings.
TUG1 was similarly discovered to be rapidly up-regulated in response to RS, according to Kondo and Suzuki. They discovered substantial DNA damage and cell death when they lowered TUG1 expression in cancer cells. “It was exciting to see the rapid increase in expression of TUG1 in response to replication stress,” Dr. Suzuki remarked. “Normally, proteins take several hours or more to increase in response to stimuli, but RNA can be synthesized quickly,” she explains. The fact that TUG1, an RNA molecule, increases immediately in response to replication stress suggests that crucial conditions must be addressed quickly.”