As specialists gather new bits of knowledge into the powerful inward universe of the human resistance framework, it has become progressively certain that mitochondria are the basic controllers of how our bodies respond to infection.
Beyond their traditional role as “forces to be reckoned with of the cell,” mitochondria play critical roles in the lives — and, crucially, deaths — of cells by directing irritation and antimicrobial guards.This implies transformations in mitochondrial-related qualities could influence the resistant framework’s capacity to fend off illness or trigger an over-reaction, causing malignant growth or fiery issues like Crohn’s sickness.
Despite the fact that there is a developing understanding of how mitochondria control the invulnerable framework, how they do it is still moderately obscure. Sorting out how mitochondrial transformations upset insusceptible reactions could be the way to understanding instruments for sicknesses like tuberculosis, uncleanliness, and Parkinson’s infection, and possibly pave the way for new therapies.
“Mutations in the protein LRRK2 have been linked to a number of major human disorders that appear to have little in common—disease, Parkinson’s leprosy, inflammatory bowel and Crohn’s disease, and cancer,”
Kristin L. Patrick from the Texas A&M University School of Medicine
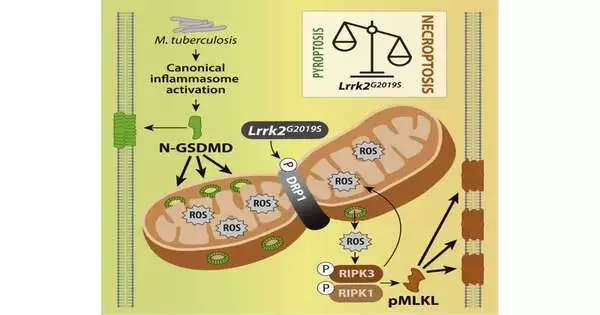
A group of specialists led by Robert O. Watson and Kristin L. Patrick from the Texas A&M College Institute of Medication recently revealed a piece of the secret in a study published in the journal Cell.Watson and Patrick concentrated on transformations in the protein LRRK2 in macrophages, or white platelets, from creature models. At the point when the transformed macrophages were presented to the microbes that cause tuberculosis, the mitochondria started another kind of cell demise, changing from pyroptosis to necroptosis.
When cells kick the bucket from necroptosis, they discharge compound signals that cause a forceful, provocative, insusceptible reaction. For the creature models with LRRK2 changes, the change to necroptotic cell demise made them more defenseless to tuberculosis contamination and set off unnecessary irritation in the tainted tissues, causing more terrible results.
Although the review zeroed in basically on macrophages and tuberculosis contamination, the newfound sickness system might have a lot more extensive ramifications.
“Transformations in the protein LRRK2 have been related to numerous significant human sicknesses that, at face value, have next to no to do with one another — Parkinson’s illness, uncleanliness, provocative entrail, Crohn’s infection, and malignant growth,” Patrick said. “Our work exhibits that a typical change in LRRK2 triggers another kind of cell death that results in hyperinflammation in response to contamination. Cell demise and aggravation could be what interfaces LRRK2 to this multitude of divergent human sicknesses. “
The group’s revelation has potential true applications that could give genuinely necessary help to patients, and Patrick and Watson are prepared to plunge into the following period of examination by testing their hypothesis by utilizing LRRK2 inhibitors previously created by drug organizations.
“Our exploration has situated us to consume these medications and study them with regards to the resistant reaction,” Patrick said. “We are going to set out on examinations to see whether sedating LRRK2, as well as different proteins engaged with this new cell-passing pathway, could further develop tuberculosis contamination results.”
The group’s revelation features the basic job of essential science in the seat-to-bedside pathway for growing new treatment choices for patients experiencing life-changing sicknesses. In the most natural-sounding way for Patrick, “It’s energizing to perceive how essential science research that dives profound into how atoms work can distinguish startling associations between apparently irrelevant illnesses and open up new roads for helpful mediation.”
More information: Chi G. Weindel et al, Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis, Cell (2022). DOI: 10.1016/j.cell.2022.06.038
Journal information: Cell