Because of their exceptional photoelectric properties, halide perovskites have been emerging as promising photocatalytic materials for H2-advancement from water. In any case, the absence of appropriate receptive surfaces extraordinarily ruins the photocatalytic capability of these captivating mixtures. In this work, Mo2C nanoparticles have been secured onto methylammonium lead iodide (MAPbI3) as a non-respectable metal cocatalyst to advance H2-development responses.
The group distributed their work on May 28 in Energy Material Advances.
“Photocatalytic water dividing has been considered as a promising way to store sunlight-based energy as hydrogen energy,” said paper creator Xiaoxiang Xu, teacher with the Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University. “At present, halide perovskites have acquired extraordinary interest as photocatalysts for H2-development responses, yet they are typically lacking in surface receptive locales where photocarriers can’t be immediately moved for surface redox responses.”
Xu made sense of the fact that the very acidic climate expected to settle halide perovskites in a watery arrangement limits the decisions of H2-advancement cocatalyst that can be saved.
“The noble-metal cocatalyst Pt was introduced to accelerate H2-evolution reactions, but it is still unsatisfactory possibly because to the poor Pt/halide perovskite interfaces. As an alternative to noble-metal cocatalysts, robust non-noble-metal-based cocatalysts have garnered substantial interest.”
Xiaoxiang Xu, professor with the Shanghai Key Lab of Chemical Assessment and Sustainability
Xu said. “The respectable metal cocatalyst Pt has been acquainted with advanced H2-development responses, however, is as yet unsuitable, most likely because of the unfortunate Pt/halide perovskite interfaces.” “Hearty non-honorable metal-based cocatalysts stand out as options in contrast to respectable metal cocatalysts.”
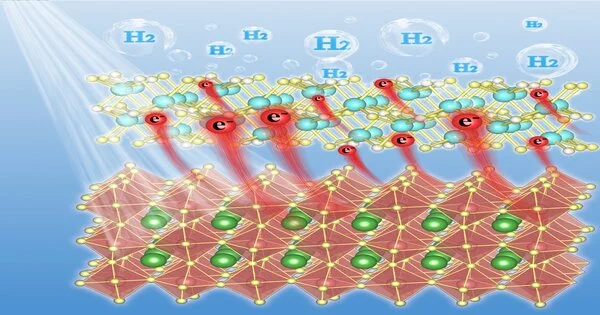
Be that as it may, their associations with halide perovskites are, for the most part, extremely feeble, frequently because of primary confounding in this way, keeping quick charge relocations from halide perovskites to these cocatalysts. As indicated by Xu, Mo2C, a promising minimal expense electrocatalyst for H2-development response, shows fantastic electrocatalytic movement over a wide pH range, in this manner filling in as a potential competitor cocatalyst for photocatalytic H2-development.
Xu said that Mo2C is a rare example of a mixture that has steady areas of strength for in, making it a magnificent option cocatalyst for halide perovskites, which are balanced out areas of strength for in during photocatalytic responses, for example, HBr and HI watery arrangement.
Xu said, “The contrary Zeta possibilities somewhere in the range of Mo2C and MAPbI3 guarantee firm interconnections between these mixtures.” “In this paper, we outlined ongoing examination speeding up H2-development over MAPbI3 with a non-honorable metal cocatalyst Mo2C under noticeable light.”
Xu said, “We have effectively stacked Mo2C nanoparticles onto MAPbI3 by an electrostatic-gathering strategy to create Mo2C@MAPbI3 composites.”
According to Xu, the Mo2C nanoparticles are homogeneously and immovably secured at the outer layer of MAPbI3. “On account of major areas of strength for somewhere in the range of Mo2C and MAPbI3, Mo2C@MAPbI3 composites display unrivaled photocatalytic movement for H2-development from water, which plainly outperforms perfect MAPbI3 and Pt-saved MAPbI3 under similar testing conditions.”
Xu said that further examination proposes that Mo2C nanoparticles work with charge division in MAPbI3 as well as significantly speed up interfacial charge movement for water decrease responses. “These findings validate the Mo2C as a productive non-respectable metal cocatalyst for halide perovskite photocatalysts that work in a highly acidic environment.”