Ocean aging, warm-blooded animal breath, and splash plan all depend on science that occurs at air-water interfaces. In another assessment, scientists from the Division of Energy’s Lawrence Berkeley Public Lab (Berkeley Lab) have tracked down which pathway carbon dioxide (CO2) iotas follow on their way from the environment into water, and it’s not the one that they expected.
The oceans ingest commonly 30% of all anthropogenic CO2 transmissions. In water, the CO2 structures are carbonic and destructive, changing the marine environment in ways that are perilous for some sea life. In our bodies, air that crosses the wet movies covering our nasal entries influences the pH of our blood.
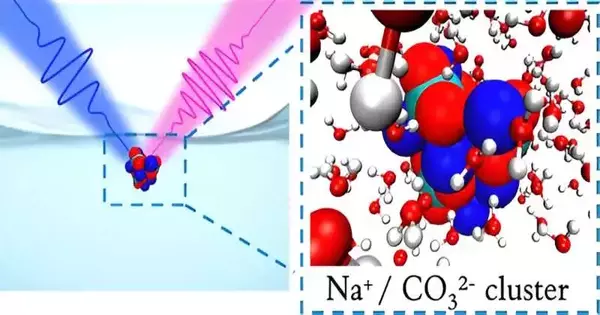
However, the very way that the local science changes truly depends on how the split-up CO2 disconnects into two particular particles with different charges—duplicately charged carbonate and independently charged bicarbonate—near the liquid surface. Berkeley Lab experts, as of now, show a better combination of carbonate at air-water interfaces, where they expected to find more bicarbonate.
“The carbon cycle of the Earth, as well as the respiration cycle of mammals, explicitly involve the dissolution of CO2 at the water surface and its transformation into bicarbonate and carbonate ions. Understanding interactions at the air-water interface will provide further light on these crucial processes.”
Jin Qian, a researcher who contributed the theoretical part of the work.
“The carbon example of Earth, as well as the breath example of warm-blooded animals, unequivocally incorporates the deterioration of CO2 at the water surface and its change into bicarbonate and carbonate particles. Understanding reactions at the air-water association point will furthermore edify these vitally huge cycles,” said Jin Qian, an expert who contributed the speculative piece of the work definite in the Journal of the American Compound Society. Qian is a staff scientist in the Compound Sciences Division at Berkeley Lab.
The substance processes that occur at a liquid-air association point are often undeniable from the very ones that occur when looking at mass liquids. Perusing the material, old-style speculations show that carbonate should remain in the mass liquid while bicarbonate should accumulate at the surface, yet an unequivocal cognizance of the pathways of the two particles has stayed foggy. Since the external layer of a response includes only a minute piece of its finished volume, assessing molecule obsessions there is problematic.
“Not only is the sign very weak, yet it ought to be disengaged from the much greater mass response of the structure,” got a handle on Richard Saykally, an educator in UC Berkeley’s Part of Science who drove the work. Saykally is a senior-ranking staff specialist in the Substance Sciences Division at Berkeley Lab.
Saykally and his accomplices used gadgets, especially those expected to measure delicate compound signs on liquid surfaces. The system, called significant UV second consonant age spectroscopy (DUV-SHG), directly tests particles at liquid places of connection.
“We can now measure the general surface populations of carbonate and bicarbonate, as well as thermodynamic information concerning their surface prejudices,” said Shane Devlin, a postdoctoral researcher at Berkeley Lab and lead maker of the survey. The gathering found that carbonate showed significantly more grounded inclination to adhere to the surface than did bicarbonate.
To get a handle on this especially unexpected approach to acting, the researchers went to speculative gadgets. Tod Pascal and his accomplices at UC San Diego ran automatic encounters to fathom how carbonate and bicarbonate particle structures pack, a cycle that was conceivable and responsible for their different concentrations at the surface and in the mass liquid.
They saw that, while packing was an ideal collaboration for carbonate, it was not such a huge amount for bicarbonate. To further get a handle on the spectroscopy discernments, Qian and her social event ran propagations using the Perlmutter structure at the Public Energy Investigation Consistent Figuring Spot (NERSC), a DOE client office at Berkeley Lab. They cultivated a procedure that involved calculations of the ridiculous fingerprints of carbonate and bicarbonate in a very immense district at the liquid-air interface.
The entertainments certified that carbonate truly does, certainly, show a much more grounded tendency for the air-water interface. It came about in view of the strong coordination of carbonate with sodium particles, which provoked unbiased gatherings of particles that then became attracted to the surface.
“This is the point at which our computational technique has first been used in a sensible applicational setting, focusing on the air-liquid association point containing around 1,000 particles,” says Qian.
While astounding, the assessment could have clear repercussions. The ocean’s surface is where air and water mix, provoking the improvement of shower drops, which expect a major job in the overall environment and barometrical models.
As the level of air CO2 continues to rise, the extent of carbonate and bicarbonate anions at the surface will most likely move, which will accordingly affect the study of marine shower drops. Understanding the normal impact of extended carbonate obsessions in fume sprayers is critical for scientists who are endeavoring to expect ecological change.
Besides, bicarbonate is a tolerably delicate molecule and can go about as a physiological pad that helps our blood and tissues stay aware of real science and metabolic capacity. On the other hand, carbonate is basically a solid area that is beyond any reasonable amount to try and think about filling in as a support. Understanding how these harmonies shift could be critical for a thorough portrayal of breath in warm-blooded animals.
“The interfacial approach to the actions of these species and cycles likewise clearly impacts both geophysical and natural cycles. The revelations of this study will spike future undertakings, highlighting the consequences for marine nature,” said Saykally.
More information: Shane W. Devlin et al. Agglomeration Drives the Reversed Fractionation of Aqueous Carbonate and Bicarbonate at the Air-Water Interface, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c05093