To meet the energy demands of next-generation electronics and electric vehicles, lithium-ion batteries with high-energy-density cathodes are required. However, at high voltages, the battery electrolyte decomposes excessively, compromising cathode performance. To address this, scientists have developed a bio-based, non-toxic additive material that stabilizes the cathode by forming a passivation layer on its surface and inhibiting its decomposition. The novel compound, which is eco-friendly and low-cost, may encourage greater use of bio-based resources.
Lithium-ion (Li-ion) batteries with high energy density are essential for powering electric and hybrid vehicles, next-generation electronics, and power grids. These Li-ion batteries have transition metal oxide cathodes with high energy density. The LiNi1/3Mn1/3Co1/3O2 cathode has been shown to deliver the best performance at a high potential of 4.5 V versus Li/Li+ with high reversible capacity among numerous investigated potential materials.
At such high potentials, however, the carbonate species in commercial electrolytes, ethylene carbonate, and diethyl carbonate, undergo excessive oxidative decomposition. As a result, a thick cathode electrolyte interphase (CEI) layer forms on the cathode surface, severely compromising its performance. As a result, researchers have investigated electrolyte additives as a means of limiting performance degradation by masking and stabilizing the cathode surface. However, the current options pose safety and environmental risks.
This study focuses on novel microbial metabolites, particularly the unique pyrazine-derived diamine DMBAP from the gene cluster of Pseudomonas fluorescens SBW25. Its role as an electrolyte additive could impact the fields of sustainability and smart-cell industry.
Prof. Takaya
A team of researchers led by Professor Noriyoshi Matsumi of Japan Advanced Institute of Science and Technology (JAIST) recently microbially synthesized 2,5-dimethyl-3,6-bis(4-aminobenzyl)pyrazine (DMBAP), a bio-based compound, as a potential additive for stabilizing the LiNi1/3Mn1/3Co1/3O2 cathodes. What distinguishes their approach is that, unlike existing additives, DMBAP is sustainable, eco-friendly, cost-effective, and non-toxic.
Former Senior Lecturer Rajashekar Badam, Postdoctoral Research Fellow Agman Gupta, and Doctoral Course Student Noriyuki Takamori from JAIST joined Professor Naoki Takaya, Assistant Professor Shunsuke Masuo, and Former Graduate Student Hajime Minakawa from the University of Tsukuba in Japan. Their findings have been published in the journal Scientific Reports.
“Although biomass-derived materials attract both researchers and society in general, their applications in electric devices, including lithium-ion batteries, are still limited. This study focuses on novel microbial metabolites, particularly the unique pyrazine-derived diamine DMBAP from the gene cluster of Pseudomonas fluorescens SBW25, discovered in collaboration with Prof. Masuo. Its role as an electrolyte additive could impact the fields of sustainability and the smart-cell industry,” explains Prof. Takaya, speaking of the motivation behind the study.
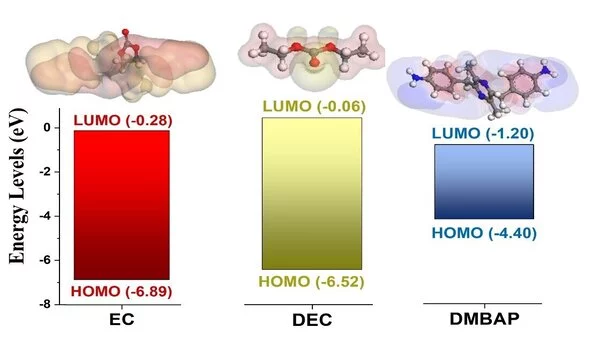
An initial theoretical evaluation revealed that the highest occupied molecular orbital (HOMO) of the DMBAP molecule was located at a higher position compared to a general-purpose electrolyte. This allowed it to be oxidized easily at the cathode surface and form a protective layer over it. In addition, the diamine in DMBAP prevented the dissolution of CEI.
In addition, the team performed a detailed electrochemical evaluation of DMBAP for further investigation. Linear sweep voltammetry confirmed the HOMO band energy, while X-ray photoelectron spectroscopy revealed CN=C peaks indicative of oxidative electropolymerization. Cyclic voltammetry and charge-discharge studies revealed that the DMBAP additive improved the battery’s rate capability, cyclic stability, coulombic efficiency, and capacity retention by stabilizing the LiNi1/3Mn1/3Co1/3O2 cathode. Experiments with dynamic electrochemical impedance spectroscopy also revealed the formation of a low interfacial resistance CEI.
Based on these findings, the researchers concluded that the DMBAP underwent sacrificial oxidative decomposition, resulting in the formation of organic passivation armor on the cathode surface. As a result, excessive electrolyte degradation was limited, and the structure of transition metal oxides on the cathode was stabilized. This virtuous phenomenon effectively raises the operating potential window of the LiNi1/3Mn1/3Co1/3O2 cathode to 4.5 V versus Li/Li+. Furthermore, DMBAP had a significant stabilizing effect on the battery system in both half-cell and full-cell configurations.
“DMBAP, a pyrazine-amine compound produced by microbes, will improve the performance of lithium-ion secondary batteries used in next-generation electric vehicles and drones. It will also encourage the greater use of bio-based resources in the massive automotive industry. Furthermore, bio-based materials for energy storage devices will reduce CO2 emissions twice: during manufacturing and operation” Prof. Matsumi discusses the long-term benefits of their work.