There’s a bouncer in everybody. Toxins, pathogens, and other undesirables that can sabotage the brain’s precious gray matter are expelled from the body through the blood-brain barrier, a layer of cells that exists between blood vessels and the rest of the brain.
There are a number of situations that could occur if the bouncer isn’t paying attention and a rowdy group enters. Cancer cells that break through the barrier can grow into tumors, and multiple sclerosis can happen when too many white blood cells break through the barrier and attack the protective layer of brain nerves, making it hard for them to communicate with the rest of the body.
“Being able to seal off the barrier has been a long sought-after goal in medicine,” stated Calvin Kuo, MD, Ph.D., the Maureen Lyles D’Ambrogio Professor and a professor of hematology. “A leaky blood-brain barrier is a common pathway for a lot of brain diseases.”
“Because a leaky blood-brain barrier is a common pathway for many brain diseases, the ability to seal off the barrier has long been a sought-after goal in medicine.”
Calvin Kuo, MD, Ph.D., the Maureen Lyles D’Ambrogio Professor and a professor of hematology.
Kuo says that there isn’t enough research on how to fix the blood-brain barrier. However, a treatment that may be helpful in restoring the barrier to its normal function is described in a recent paper that he and colleagues led. The Nature Communications-published paper is by Kuo, the senior author.
“A new class of therapeutic molecules that can be used to treat blood-brain barrier leakage has been evaluated by us; before, there were no treatments specifically targeting the blood-brain barrier,” Kuo stated.
WNT signaling, a communication pathway that cells use to promote tissue regeneration and wound healing, was the focus of the researchers’ initial investigation. By encouraging cell-to-cell communication along the blood vessels that run through the brain, WNT signaling helps to maintain the blood-brain barrier.
Kuo stated, “There is a great deal of historical data that indicates that the WNT signaling pathway would be important for maintaining the blood-brain barrier.” The opportunity presented itself to test a novel WNT signaling pathway that would activate signaling across the blood-brain barrier by binding to a receptor called frizzled in a highly selective manner.”
Since mouse mutations in the frizzled gene cause abnormalities in the blood-brain barrier, researchers have been focusing on frizzled, a protein receptor that initiates the WNT pathway, for blood-brain barrier therapies.
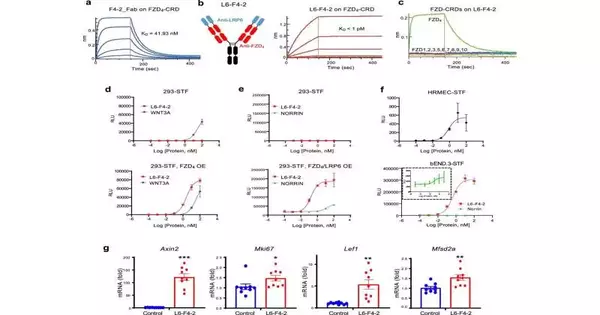
How it is made Because many different molecules bind to frizzled protein receptors, the researchers narrowed their search for a potential therapeutic molecule to those that specifically target brain blood vessel-lining cells.
In the laboratory, Chris Garcia, Ph.D., a Younger Family Professor of molecular and cellular physiology, created prototype therapeutic WNT pathway molecules, including a molecule that activates the frizzled receptor FZD4. L6-F4-2, a FZD4-binding molecule that activates WNT signaling 100 times more effectively than other FZD4 binders, was developed by collaborators at a research company based on the work of Garcia and Kuo.
A stronger blood-brain barrier was observed when the group, which included research scientist Jie Ding, increased the rate at which WNT signaling was activated.
Keeping the bouncer busy The researchers wanted to find out if L6-F4-2 can successfully replace the natural molecular key for frizzled and what happens when it is missing. As a result, they turned to Norrie disease, a genetic condition that causes the blood-retinal barrier to leak.
The blood-brain barrier serves the brain in the same way that the blood-retinal barrier serves the eye. In Norrie disease, the layer of light-sensitive cells in the back of the eye is hindered in its development of blood vessels, resulting in leaky connections, improper development, and blindness.
Mutations in the NDP gene cause Norrie disease. The NDP gene tells how to make a protein called Norrin, which is the key that opens the FZD4 receptor lock and turns it on. The key is missing, the gene is inactive, and the mice in the study are blind due to a leaky barrier. L6-F4-2, which the researchers refer to as a surrogate, was used to replace the Norrin protein that was missing.
The mice’s blood-retinal layer was restored after the Norrin protein was replaced by L6-F4-2. The blood vessels were imaged and found to be denser and less leaky than before treatment, so the researchers knew this. The researchers also demonstrated that L6-F4-2 replaced Norrin and activated WNT signaling in the blood-brain barrier surrounding the mice’s cerebellum, which is a region that is responsible for muscle coordination.
Next, the researchers wanted to investigate a more prevalent human condition called ischemic stroke, in which blood, fluid, and inflammatory proteins involved in cellular communication can leak into the brain due to damage to blood vessels and the blood-brain barrier. When compared to mice with strokes that were not treated, they discovered that mice treated with L6-F4-2 had better survival rates and less severe strokes. Importantly, after a stroke, brain blood vessel leakage was reversed by L6-F4-2.
Stroke survival rates were higher in L6-F4-2-treated mice than in untreated ones.
Drugs that activate FZD receptors and the WNT signaling pathway have the potential to restore the blood-brain barrier in mice, as demonstrated by this discovery.
Kuo is excited about the potential for a treatment for a number of other neurological diseases, such as Alzheimer’s, multiple sclerosis, and brain tumors, because a number of disorders originate from dysfunction of the blood-brain barrier.
“We trust this will be an initial move toward fostering another age of medications that can fix the blood-cerebrum obstruction, utilizing a totally different procedure and sub-atomic objective than current prescriptions,” Kuo said.
More information: Jie Ding et al, Therapeutic blood-brain barrier modulation and stroke treatment by a bioengineered FZD4-selective WNT surrogate in mice, Nature Communications (2023). DOI: 10.1038/s41467-023-37689-1