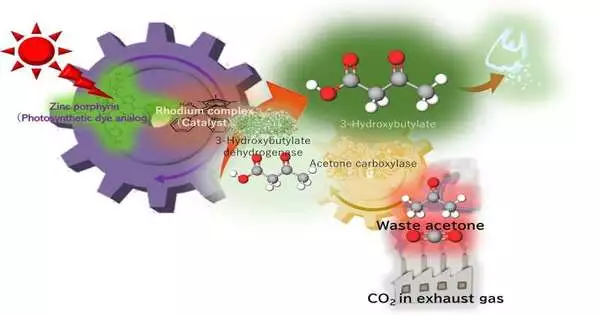
Scientists from Osaka Metropolitan University have created a method that successfully converts more than 60% of waste acetone into 3-hydroxybutyrate, a substance used to create biodegradable plastic, using artificial photosynthesis. Low-concentration CO2, which is exhaust gas, and light for a full day, which is sunlight, were used to get the results.
The researchers predict that this novel method of making biodegradable plastic could not only lower CO2 emissions but also offer a way to reuse acetone from industrial and laboratory waste. In the journal Green Chemistry, their findings have been presented.
Made from 3-hydroxybutyrate as a precursor, poly-3-hydroxybutyrate is a biodegradable plastic that is a sturdy, water-resistant polyester that is frequently used in packaging materials. In earlier studies, a research group under the direction of Professor Yutaka Amao from the Research Center for Artificial Photosynthesis at Osaka Metropolitan University discovered that 3-hydroxybutyrate could be synthesized from CO2 and acetone with high efficiency, though this was only proven at higher concentrations of CO2 or sodium bicarbonate.
“We focused on the relevance of employing CO2 produced by exhaust gas from thermal power plants and other sources to illustrate the practical application of artificial photosynthesis,”
Professor Yutaka Amao from the Research Center for Artificial Photosynthesis at Osaka Metropolitan University.
Ink waste from permanent markers and low CO2 concentrations, which are exhaust gases from steel mills, chemical plants, and power plants, were the focus of this new study. Acetone is a relatively cheap and risk-free chemical that is employed in a variety of laboratory settings, either for reactions or as a cleaning agent, and that generates waste acetone. Utilizing artificial photosynthesis, which is powered by light comparable to sunlight, acetone and CO2 served as the raw materials to create 3-hydroxybutyrate.
We concentrated on the significance of utilizing CO2 produced by exhaust gas from thermal power plants and other sources to illustrate the real-world use of artificial photosynthesis, according to Professor Amao.
After 24 hours, 3-hydroxybutyrate had successfully converted more than 60% of the acetone. The goal, according to Professor Amao, is to advance artificial photosynthesis technology so that it can use acetone from liquid waste as well as exhaust gas from the lab as raw materials.
More information: Yu Kita et al, Visible-light-driven 3-hydroxybutyrate production from acetone and low concentrations of CO2 with a system of hybridized photocatalytic NADH regeneration and multi-biocatalysts, Green Chemistry (2023). DOI: 10.1039/D3GC00247K