Even with fast-charging methods, it takes at least 30 minutes to charge an electric vehicle, which typically takes 10 hours or more. That is, assuming there is a charging station available. In the event that we could charge electric vehicles as fast as we refuel fuel-controlled vehicles, it could assist in mitigating the deficiency of EV charging stations.
The ability of the anode material to store lithium ions determines the efficiency of lithium-ion batteries, which are used in electric vehicles. A new anode material was recently developed by a team of researchers under the direction of Professor Won Bae Kim, who is from the Department of Chemical Engineering and the Graduate Institute of Ferrous and Energy Materials Technology at Pohang University of Science and Technology (POSTECH, President Moo Hwan Kim).
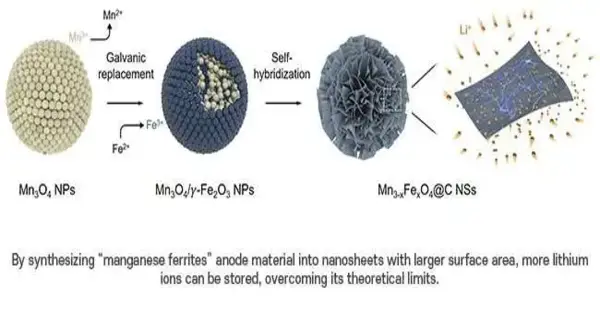
Manganese ferrites (Mn3-xFexO4) nanosheets were synthesized by his team, which also included Ph.D. candidates Song Kyu Kang and Minho Kim from the Department of Chemical Engineering. They used a novel self-hybridization technique that involved a straightforward galvanic replacement-derived process.
“By using rational design with surface alteration using electron spin, we have provided a new understanding of how to overcome the electrochemical limitations of conventional anode materials and increase battery capacity.”
Professor Won Bae Kim, who spearheaded the research,
This ground-breaking method enables an electric vehicle to be charged in as little as six minutes and increases storage capacity approximately 1.5 times above the theoretical limit. As a front-cover paper in Advanced Functional Materials, the research was acknowledged for its excellence.
A novel approach to the synthesis of manganese ferrites, an anode material renowned for its superior lithium-ion storage capacity and ferromagnetic properties, was developed in this study. Initialy, a galvanic substitution response occurred in an answer of manganese oxide blended in with iron, prompting a heterostructure compound with manganese oxide inside and iron oxide outside.
In addition, the team developed manganese ferrites with expanded surface areas in nanometer-thick sheets using a hydrothermal technique. By utilizing electrons with a high spin polarization, this method significantly increased the storage capacity for a large number of lithium ions. The team was able to effectively exceed the manganese ferrites anode material’s theoretical capacity by more than 50% thanks to this innovation.
The battery’s charging speed was improved by increasing the surface area of the anode material, which made it easier for a large number of lithium ions to move simultaneously. A battery with a capacity comparable to that found in EVs currently on the market can be charged and discharged in just six minutes, according to the findings of the experiments. The challenging synthesis procedure has been improved in this study to significantly accelerate the battery charging process and achieve a breakthrough in the material’s theoretical capacity.
“We have offered a new understanding of how to overcome the electrochemical limitations of conventional anode materials and increase battery capacity by applying rational design with surface alteration using electron spin,” said Professor Won Bae Kim, who was in charge of the research. He expressed optimism that this development could result in shorter recharging times for electric vehicles and improved battery durability.
More information: Song Kyu Kang et al, Exceeding Theoretical Capacity in Exfoliated Ultrathin Manganese Ferrite Nanosheets via Galvanic Replacement‐Derived Self‐Hybridization for Fast Rechargeable Lithium‐Ion Batteries, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202300143