High intensity focused ultrasound (HIFU) is a treatment that uses high frequency sound waves to kill cancer cells. Because HIFU does not pass through solid bone or air, it is not appropriate for all cancers. This treatment is provided by a machine that emits high-frequency sound waves. These waves direct a powerful beam to a specific area of a cancer. The high-intensity ultrasound beam is directed directly at the cancer, heating it up and killing it.
While low-intensity ultrasound has been used as a medical imaging tool since the 1950s, experts are now using and expanding models that help capture how high-intensity focused ultrasound (HIFU) can work on a cellular level. Researchers are getting closer to making the use of acoustic waves to target and destroy cancerous tumors a reality.
While doctors have used low-intensity ultrasound as a medical imaging tool since the 1950s, researchers at the University of Waterloo are developing and refining models to better understand how high-intensity focused ultrasound (HIFU) works on a cellular level.
We develop the mathematical models that engineers and doctors use to put HIFU into practice. We discovered that by running mathematical models in computer simulations, fundamental problems in technology can be solved without putting actual patients at risk.
Siv Sivaloganathan
The study, led by Siv Sivaloganathan, an applied mathematician and researcher at the Fields Institute’s Centre for Math Medicine, discovered that by running mathematical models in computer simulations, fundamental problems in technology can be solved without putting actual patients at risk.
Sivaloganathan and his graduate students June Murley and Kevin Jiang, as well as postdoctoral fellow Maryam Ghasemi, develop the mathematical models that engineers and doctors use to put HIFU into practice. He stated that his colleagues in other fields are interested in the same issues, “but we’re approaching it from different angles.”
“My contribution will be to use mathematics and computer simulations to create a solid model that others can use in labs or clinical settings. And, while the models are not nearly as complex as human organs and tissue, they provide a significant head start for clinical trials.”
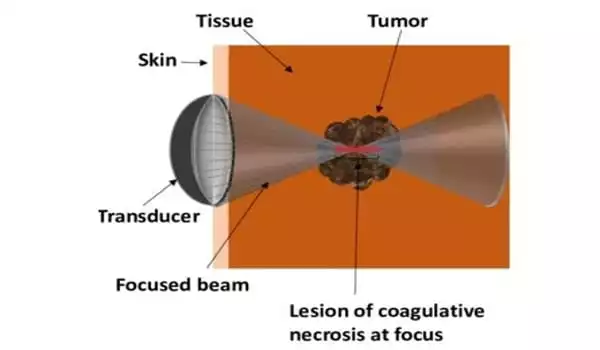
One of the challenges that Sivaloganathan is currently working to overcome is the fact that while HIFU targets cancers, it also poses risks to healthy tissue. When HIFU is used to destroy tumors or cancerous lesions, the hope is that healthy tissue is spared. The same is true when intense acoustic waves are focused on a tumor on the bone, where a lot of heat energy is released. Sivaloganathan and his colleagues are investigating how heat dissipates and whether it harms bone marrow.
Engineers who are developing the physical technology are collaborating with Sivaloganathan, as are medical doctors, particularly James Drake, chief surgeon at Hospital for Sick Children, who is investigating the practical application of HIFU in clinical settings.
Sivaloganathan believes that HIFU will significantly improve cancer treatments as well as other medical procedures and treatments. HIFU is already being used in the treatment of some types of prostate cancer.
“It’s an area that I believe will take center stage in clinical medicine,” he predicted. “It does not have the drawbacks of radiation therapy or chemotherapy. Other than the effect of heat, which we are currently working on, there are no side effects. It can also be used to break up blood clots and even to administer drugs.”
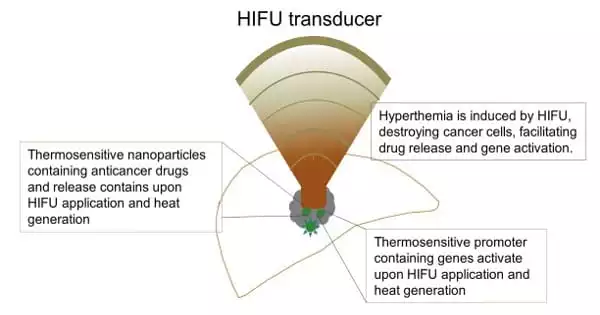
According to Sivaloganathan, HIFU has potential beyond its use as a cancer therapy or diagnostic tool; it can also be used for clot lysis and drug delivery when combined with nanoparticles or via the sonoporation mechanism – a process that introduces pores into the cell membrane.
HIFU can be used to break up clots in blood vessels by inducing the physical phenomenon of unstable or “transient” cavitation, which involves the sudden formation and collapse of bubbles, resulting in energy release that is harmful to the cell. Stable cavitation, on the other hand, has the potential to improve therapeutic delivery to the brain via the blood–brain barrier by increasing cell membrane permeability and drug release via the generation of oscillating bubbles that do not collapse.