A group of researchers, driven by Xiling Shen, Ph.D., Chief Scientific Officer and Professor at the Terasaki Institute for Biomedical Innovation (TIBI), has arrived at new levels in a quiet model turn of events. They have created better strategies for producing miniature organospheres (MOS) and have shown that these MOS have prevalent capacities for various clinical purposes. As recorded in a new paper in Stem Cell Reports, their MOS can be utilized as quiet symbols for studies including direct popular disease, safe cell entrance, and high-throughput helpful medication screening, something not possible with regular patient-determined models.
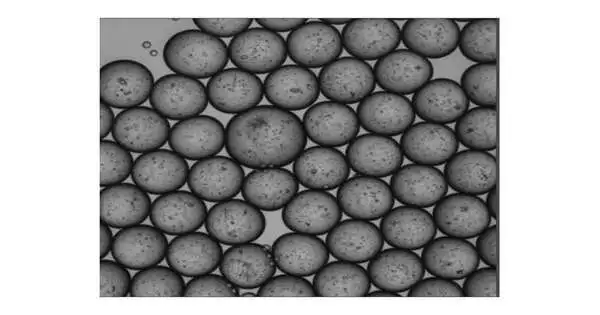
Dr. Shen’s group has created an emulsion microfluidic innovation for making MOS, which are small, nanoliter-sized basal film emulsion (BME) drops made out of tissue cell blends that can be produced at a fast speed by a robotized gadget. After the beads are created, a creative film demulsification process extracts a large amount of oil, leaving behind a large number of gooey, consistently measured drops with small three-dimensional tissue structures.
The group proceeded to show novel MOS abilities and elements in a few first-of-their-sort tests. They had the option to demonstrate the way that the MOS could be made from a wide range of tissue sources and that the resultant MOS had maintenance of histopathological morphology, limit with respect to separation and hereditary articulation, and the capacity to be frozen and sub-refined, as in regular organoids.
“Dr. Shen and his team continue to develop and enhance the MOS technology and highlight its adaptability, not just as a physiological model for evaluating prospective customized treatments, but for disease investigations and a range of other uses as well,”
Ali Khademhosseini, Ph.D.
Tests were conducted to test the capacity to taint MOS with infections. As with regular organoids, MOS can be straightforwardly tainted with infections without the expulsion and suspension of cells from its encompassing BME platform, thus restating the course of viral disease in the host tissue. Dr. Shen’s group had the option to make a MOS map book of human respiratory and stomach-related tissues from patient dissections and taint them with SARS-CoV-2 infections, followed by drug screening to recognize drugs that block viral disease and replication inside those tissues.
MOS likewise gives a novel stage to examining and creating safe cell treatment. For example, cancer inferred MOS permitted adequate entrance by helpful safe T-cells, for example, CAR-T, empowering a clever T-cell power measure to survey growth killing by the designed T-cells. Such a model would be profoundly helpful in examining cancer responsiveness and in creating hostile-to-growth safe cell treatments.
MOS could also be combined with deep pick up imaging examination for quick medication testing of small and diverse clinical cancer biopsies.Also, the calculation had the option to recognize cytotoxic versus cytostatic drug impacts and medication safe clones that will lead to later backslides. This notable ability will prepare MOS to be utilized in the center to illuminate helpful choices.
“Dr. Shen and his group proceeded to refine and enhance the MOS innovation and to highlight its flexibility, not just as a physiological model for screening likely customized medicines, but for illness studies and various different applications too,” said Ali Khadem hosseini, Ph.D., TIBI’s Director and CEO. “It seems to be the rush of representing things to come for accuracy medication.”
More information: Zhaohui Wang et al, Rapid tissue prototyping with micro-organospheres, Stem Cell Reports (2022). DOI: 10.1016/j.stemcr.2022.07.016
Journal information: Stem Cell Reports