Substance partition processes are fundamental in the assembling of numerous items, from fuel to bourbon. Such cycles are vivaciously exorbitant, accounting for roughly 10–15 percent of global energy consumption.Specifically, the utilization of supposed “warm partition processes,” for example, refining for isolating petrol-based hydrocarbons, is profoundly instilled in the compound business and has an exceptionally enormous related energy impact. Layer-based partition processes can possibly diminish such energy utilization fundamentally.
Film filtration processes remove different impurities from the air we inhale and the water we drink. Notwithstanding, film innovations for isolating hydrocarbons and other natural materials are undeniably less evolved.
Penn Engineers are growing new layers for energy-effective natural partitions by reexamining their actual design on the nanoscale.
Eduardo D. Glandt Presidential Professor of Chemical and Biomolecular Engineering Chidum Osuji and his labThe exhibition of these films was featured in a past report portraying how the design of the actual layer assisted in limiting the restricting tradeoff between selectivity and penetrability that is experienced in conventional nanofiltration layers. This innovation was likewise remembered for last year’s Y-Prize contest, and the victors have made a case for its utilization to create non-fermented brew and wine in a startup called LiberTech.
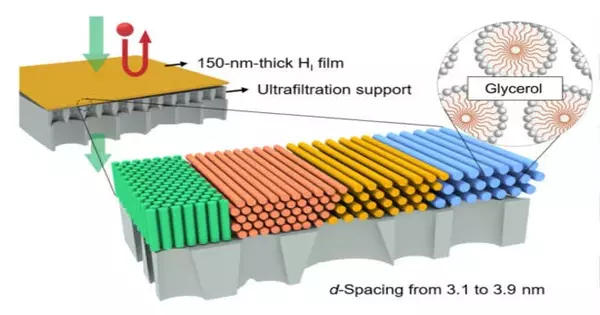
At present, Osuji’s most recent review adjusts the film for filtration in natural arrangements like ethanol and isopropyl liquor, and its self-gathering particles make it more proficient than conventional natural dissolvable nanofiltration (OSN).
The review, which was published in Science Advances, demonstrates how the uniform pores of this layer can be adjusted by varying the size or grouping of the self-gathering particles that eventually structure the material.This adaptability now opens the door for the use of this film innovation in addressing more diverse certifiable natural filtration issues.Analysts in the Osuji lab, including first creator and previous postdoctoral specialist, Yizhou Zhang; postdoctoral scientist, Dahin Kim; and graduate understudy, Ruiqi Dong; as well as Xunda Feng of Donghua University, added to this work.
“At a certain concentration in an aqueous solution, the surfactant molecules aggregate and form cylindrical rods, and then those rods will self-assemble into a hexagonal structure, yielding a gel-like material,”
Osuji lab
University of Pennsylvania is to blame.
One test the group faced was the trouble of keeping up with layer security in natural solvents with various polarities. They chose atomic species, surfactants, that showed low dissolvability in natural liquids and which could be connected together artificially to give the necessary security. The surfactants self-collect in water when they are over a specific fixation and structure a delicate gel. Such self-get together — the arrangement of an arranged state — as a component of focus is alluded to as lyotropic conduct: “lyo-” alluding to arrangement, and “-jungle” alluding to arrangement. The gels in this frame are called lyotropic mesophases.
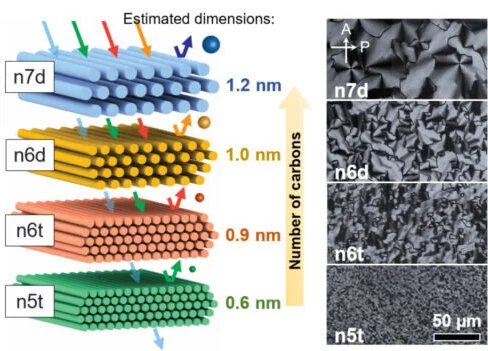
The layers created in this study were made by shaping the initially framed lyotropic mesophases of the surfactant in water, extending the delicate gel as a long film, and afterward utilizing a substance response to interface the surfactants together to shape a nanoporous polymer. The size of the pores in the polymer is set by the self-gathered design of the lyotropic mesophase.
“At a specific fixation in a fluid arrangement, the surfactant particles total and structure round and hollow bars, and afterward those poles will self-collect into a hexagonal construction, yielding a gel-like material,” says Osuji. “One of the ways in which we can control the porousness, or size of the pores in our films, is by changing the focus and size of the surfactant atoms used to make the actual layer. In this review, we controlled both of those factors to tune our pore sizes from 1.2 nanometers down to 0.6 nanometers. “
These layers are viable with natural solvents and can be customized to address different partition difficulties. Natural dissolvable nanofiltration can diminish the impression of conventional warm detachment processes. The uniform pore size of the films created here gives convincing benefits with regards to layer selectivity and, eventually, energy productivity too.
“A particular application for this innovation is in biofuel creation,” says Osuji. “The seclusion of water-miscible alcohols from bioreactors is a key stage in the assembling of ethanol and butanol biofuels.” Film detachments can reduce the energy required to separate the alcohols or energizes from the fluid medium in the reactor.The utilization of films is especially invaluable in more limited-size activities, for example, this, where refining isn’t financially savvy.
Furthermore, the assembling of numerous drug items frequently includes a few stages of union in various dissolvable conditions. Those means require the exchange of a compound moderate, starting with one dissolvable and then onto the next miscible dissolvable, making this new layer an ideal answer for drug improvement filtration needs.
Subsequent stages of their exploration include both hypothesis and practice. The group intends to foster new models for film dismissal and porousness that address the extraordinary stream of examples of arrangements through their layers as well as recognize additional future applications for their tunable innovation.