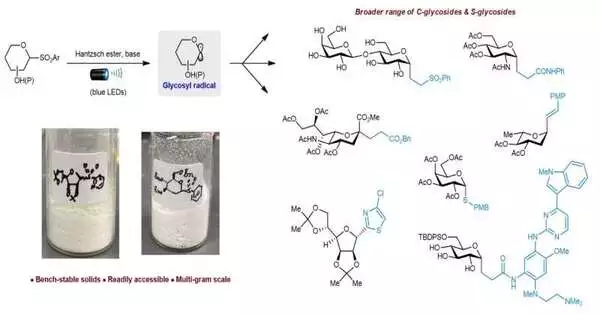
Physicists at the Public University of Singapore have developed a new method for producing therapeutically important C-glycosides and S-glycosides using an impetus and progress without metal methodology under visible light brightening at a high temperature.Their exploration shows up in Nature Blend.
Glycosides play a key role in different physiological capabilities and are tracked down in a wide assortment of normal items and engineered compounds. C-glycosides are a significant class of glycosides, which comprise a sugar-containing unit joined to a natural moiety or another sugar-containing compound through a carbon (C) bond. They have a heap of natural exercises and are basically different. One helpful method for building such items includes the immediate association of a glycosyl antecedent (giver) with a carbon-based reagent.
Nonetheless, the extent of C-glycosides that can be gotten to by utilizing right now’s detailed strategies is seriously restricted. This is due to the absence of useful glycosyl givers accessible for work with gentle C coupling. A general class of seat stable glycosyl forerunners that can be quickly blended and isolable for a wide range of applications yet are sufficiently receptive to going through quick and stereoselective cross-coupling at surrounding conditions is profoundly appealing yet subtle.
“We anticipate that this general class of glycosyl precursors, as well as their newly discovered reactivity under visible light illumination, will find widespread utility in various carbohydrate synthetic applications, enhancing efforts to discover new sugar-based therapeutics and our understanding of biological processes.”
Assistant Professor Koh Ming Joo
An exploration group led by Partner Teacher Koh Ming Joo, from the Branch of Science, Public College of Singapore, has conceived hearty systems to blend strong heteroaryl glycosyl sulfones on a multi-gram scale. The group found that these seat-stable sulfones are likewise redox-dynamic. By utilizing noticeable (blue) light brightening and a Hantzsch ester-base intricate, the scientists can enact the sulfones to create synthetically receptive glycosyl extremists.
These extremists respond promptly with different electrophiles. With this technique, they can get a more extensive scope of important C-alkyl, C-alkenyl, C-alkynyl, C-heteroaryl, and S-connected glycosides in an effective and profoundly particular way. The analysts likewise utilized bright/apparent ingestion spectroscopic and extremist clock studies to acquire experience of these changes.
Prof. Koh said, “This impetus and change sans metal strategy really beats past limits in degree, versatility, and glycosyl giver shakiness.”
“We expect this general class of glycosyl antecedents and their newfound reactivity under apparent light brightening to find broad utility in different carbohydrate engineered applications, improving endeavors towards the revelation of new sugar-based therapeutics and our understanding of natural cycles,” added Prof. Koh.
The exploration group intends to work with organizations to use these discoveries for the blend of sugar-inferred compounds.
More information: Quanquan Wang et al, Visible light activation enables desulfonylative cross-coupling of glycosyl sulfones, Nature Synthesis (2022). DOI: 10.1038/s44160-022-00162-w
Journal information: Nature Synthesis