Cell attachment atoms that interact with opposing cells maintain cell, tissue, and organ structure.Cadherins are a class of fundamental cell-bond particles for tissue development and uprightness, and imperfections in cadherin capability cause different illnesses (e.g., disease attack). Cadherin distends from the cell surface and ties another cadherin to a restricting cell to form an intervening cell bond. The cadherin-restraining interaction fundamentally contains two dimerization steps: X-dimer development and strand-trade (SS-) dimer arrangement of the extracellular spaces (ectodomains) of cadherin. Be that as it may, connections other than those including the arrangement of the X-and SS-dimers have likewise been proposed, and the exact restricting instrument of cadherin stays disputable.
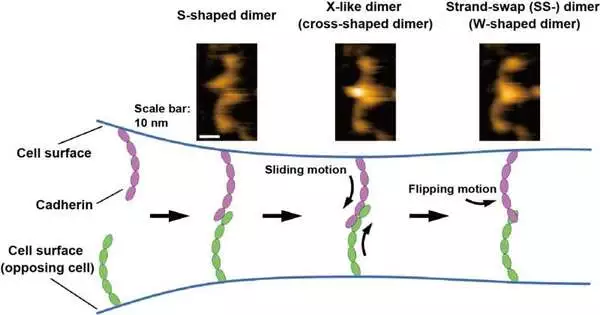
Shigetaka Nishiguchi of ExCELLS, Takayuki Uchihashi of ExCELLS and Nagoya University, and Tadaomi Furuta of Tokyo Tech applied fast nuclear power microscopy (HS-AFM) to investigate the limiting component of cadherins. HS-AFM can empower the perception of single-particle designs and elements in arrangement at the nanometer scale with a sub-second time goal by straightforwardly contacting and filtering the outer layer of proteins through a sharp-tipped test. HS-AFM uncovered that cadherins existed as various dimeric structures, which in view of their morphology might be named W-, cross-, and S-molded dimers.
Besides, the researchers led mutational and underlying demonstrating investigations and discovered that W-and cross-molded dimers are related to known SS-dimers and X-like dimers and that the S-formed dimer is a clever compliance. The limiting cycles of cadherins straightforwardly envisioned by HS-AFM likewise uncovered that the dimerization interaction is finished in somewhere around one second through transformation into the previously mentioned three sorts of dimeric structures. In light of these HS-AFM perceptions, the researchers conjectured that the limiting component progresses through the sliding movement of the S-molded dimer followed by the flipping movement of the X-dimer to frame the SS-dimer, which is believed to be the last steady cadherin dimer.
Until this point in time, the limiting component of cadherins has been primarily explored utilizing primary examinations and cell and arrangement estimations, which can break down the limiting states reflected by the enormous number of cadherins. The recently applied HS-AFM method uncovered the limiting cycles of individual cadherins at a single-particle goal, which has not been accomplished previously. HS-AFM perception will prepare for a more profound understanding of the limiting instrument of cadherins, which is significant for tissue-and organ-level association and cell bond related sicknesses.
The exploration was published in the Proceedings of the National Academy of Sciences.
More information: Multiple dimeric structures and strand-swap dimerization of E-cadherin in solution visualized by high-speed atomic force microscopy, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2208067119