Scientists at the University of California San Diego School of Medicine recently found a bunch of human quality changes that safeguard more established grown-ups against mental deterioration and dementia. In another review, published July 9, 2022 in Molecular Biology and Evolution, they center around one of these changed qualities and endeavor to follow its development — when and why it showed up in the human genome. The discoveries suggest specific tension from irresistible microorganisms like gonorrhea might have advanced the rise of this quality variation in Homo sapiens and coincidentally upheld the presence of grandparents in human culture.
For generations, most creature species’ science has advanced, frequently to the detriment of future well-being and longer life expectancy.As a matter of fact, people are one of the main animal groups known to live beyond menopause. As per the “grandma speculation,” this is on the grounds that more established ladies offer significant help in bringing up human babies and kids, who require more consideration than the young of different species. Researchers are presently attempting to comprehend what highlights of human science make this more extended term of wellbeing conceivable.
When scientists recently looked at human and chimpanzee genomes, they found that people have an extraordinary variant of the quality for CD33, a receptor communicated in safe cells. The standard CD33 receptor binds to sialic corrosive, a type of sugar found on all human cells.At the point when the invulnerable cell detects the sialic corrosive through CD33, it perceives the other cell as a component of the body and doesn’t go after it, forestalling an immune system reaction.
“Neanderthals normally have the same version as humans for most genes that are distinct in humans and chimps, so this was highly shocking to us. These findings show that the wisdom and care of healthy grandparents may have provided us with a significant evolutionary advantage over other ancient hominin species.”
Co-senior author Ajit Varki
The CD33 receptor is additionally expressed in cerebrospinal resistant cells called microglia, where it helps control neuroinflammation. Be that as it may, microglia additionally play a significant part in cleaning up harmed synapses and amyloid plaques related to Alzheimer’s sickness. Standard CD33 receptors suffocate this important microglial capability and increase the risk of dementia by focusing on the sialic acids on these cells and plaques.
This is where the new quality variation comes in. Somewhere along the developmental line, people got an extra transformed type of CD33 that is feeling the loss of the sugar-restricting site. The changed receptor no longer responds to sialic acids on harmed cells and plaques, permitting the microglia to separate them. For sure, more significant levels of this CD33 variation were freely observed to be defensive against late-beginning Alzheimer’s.
In attempting to comprehend when this quality variation previously arose, co-senior creator Ajit Varki, MD, Distinguished Professor of Medicine and Cellular and Molecular Medicine at UC San Diego School of Medicine, and partners found proof areas of strength for their determination, recommending something was driving the quality to advance surprisingly quickly. They likewise found that this specific adaptation of CD33 was absent in the genomes of Neanderthals or Denisovans, our nearest transformative family members.
“For most qualities that are different in people and chimps, Neanderthals typically have similar renditions as people, so this was truly amazing for us,” said Varki. “These discoveries propose that the insight and care of sound grandparents might have been a significant developmental benefit that we had over other old hominin species.”
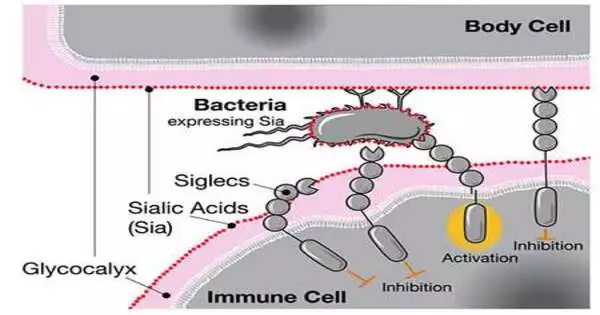
When siglecs like CD33 sense human sialic acids, they hinder the invulnerable cell’s reaction, regardless of whether those acids are situated on microbes.
Varki led the review with Pascal Gagneux, Ph.D., teacher of pathology at UC San Diego School of Medicine and teacher in the Department of Anthropology. The creators said the review gives new proof supporting the grandmother’s speculation.
In any case, developmental theory suggests that conceptive achievement, rather than post-regenerative mental wellbeing, is the primary driver of hereditary choice.So what was pushing the predominance of this transformed type of CD33 in people?
One possibility, the authors propose, is that profoundly irresistible illnesses like gonorrhea, which can impede conceptual wellbeing, may have influenced human advancement.Gonorrhea microbes coat themselves in the very sugars that CD33 receptors bind to. Like a fraud, microscopic organisms can deceive human insusceptible cells into not recognizing them as outside intruders.
The scientists recommend that the changed variant of CD33 without a sugar-restricting site arose as a human variation against such “sub-atomic mimicry” by gonorrhea and different microbes. They were sure that one of the human-explicit changes had the option to totally abrogate the cooperation between the microscopic organisms and CD33, which would permit invulnerable cells to go after the microorganisms once more.
By and large, the creators accept that people at first acquired the transformed type of CD33 to safeguard against gonorrhea during regenerative age, and this quality variation was later co-selected by the mind for its advantages against dementia.
“It is conceivable that CD33 is one of numerous qualities chosen for their endurance benefits against irresistible microorganisms right off the bat throughout everyday life, except that they are then optionally chosen for their defensive impacts against dementia and other maturing-related illnesses,” said Gagneux.
Co-creators incorporate Sudeshna Saha, Naazneen Khan, Andrea Verhagen, Aniruddha Sasmal and Sandra Diaz at UC San Diego; Troy Comi and Joshua M. Akey at Princeton University; Hai Yu and Xi Chen at UC Davis, and Martin Frank at Biognos AB.
More information: Sudeshna Saha et al, Evolution of Human-specific Alleles Protecting Cognitive Function of Grandmothers, Molecular Biology and Evolution (2022). DOI: 10.1093/molbev/msac151