Plastics are ubiquitous in our society, are tracked down in bundling, and account for more than 18% of solid waste in landfills.Large numbers of these plastics likewise advance into the seas, where they require many years to separate into pieces that can hurt natural life and the oceanic environment.
A group of scientists, led by Youthful Shin Jun, Teacher of Energy, Natural, and Compound Designing in the McKelvey School of Design at Washington College in St. Louis, examined how light separates polystyrene, a nonbiodegradable plastic from which pressing peanuts, DVD cases, and expendable utensils are made. Also, they found that nanoplastic particles can assume dynamic roles in natural frameworks. When exposed to light, the polystyrene nanoplastics accelerated the oxidation of fluid manganese particles and the formation of manganese oxide solids, which can influence the fate and transport of natural impurities in natural and designed water frameworks.
The exploration, distributed in ACS Nano on Dec. 27, 2022, showed how the photochemical response of nanoplastics to light ingestion creates peroxyl and superoxide extremists on nanoplastic surfaces and starts the oxidation of manganese into manganese oxide solids.
“Because of their increased surface area, the smaller particle size of polystyrene nanoplastics may degrade and release organic materials more easily. This liquid organic materials may swiftly create reactive oxygen species in light, facilitating manganese oxidation.”
Young-Shin Jun, Professor of Energy, Environmental & Chemical Engineering in the McKelvey School of Engineering
“As more plastic trash gathers in the common habitat, there are expanding worries about its unfriendly impacts,” said Jun, who drives the Natural Nanochemistry Lab. “However, we have generally been concerned about the jobs of nanoplastics’ actual presence rather than their dynamic jobs as reactants.””We found that such little plastic particles can more effectively connect with adjoining substances, like weighty metals and natural toxins, and can be surprisingly receptive.”
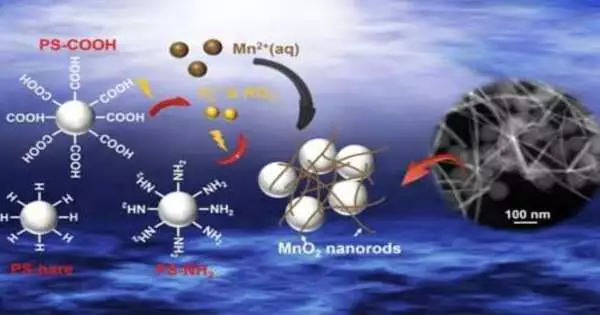
Jun and her previous understudy, Zhenwei Gao, who procured a doctorate in natural design at WashU in 2022 and is presently a postdoctoral researcher at the College of Chicago, tentatively showed that the different surface useful gatherings on polystyrene nanoplastics impacted manganese oxidation rates by impacting the age of the profoundly receptive extremists, peroxyl and superoxide revolutionaries. The creation of these receptive oxygen species from nanoplastics can imperil marine life and human wellbeing and possibly influence the portability of the nanoplastics in the climate through redox responses, which could thus adversely affect their natural remediation.
The researchers also investigated the effects of particle size on manganese oxidation using 30 nanometer, 100 nanometer, and 500 nanometer particles.The two bigger measured nanoparticles took more time to oxidize manganese than the more modest particles. At last, the nanoplastics will be encircled by recently shaped manganese oxide strands, which can make them easily amassed and can change their reactivities and transport.
“The more modest molecule size of the polystyrene nanoplastics may more effectively decay and deliver natural matter due to their bigger surface region,” Jun said. “This fragmented natural matter may rapidly generate receptive oxygen species in light and collaborate with manganese oxidation.”
“This trial work also provides valuable insights into the heterogeneous nucleation and development of manganese oxide solids on such natural substrates, which helps us interpret manganese oxide events in the climate and designed material blends,” Jun said.”These manganese solids are great foragers of redox-dynamic species and weighty metals, further influencing geochemical component redox cycling, carbon mineralization, and organic digestion systems in nature.”
Later on, Jun’s group intends to concentrate on the breakdown of assorted normal plastic sources that can deliver nanoplastics and receptive oxidizing species and explore their dynamic jobs in the oxidation of change and weighty metal particles.
More information: Zhenwei Gao et al, Oxidative Roles of Polystyrene-Based Nanoplastics in Inducing Manganese Oxide Formation under Light Illumination, ACS Nano (2022). DOI: 10.1021/acsnano.2c05803
Journal information: ACS Nano