Perovskites hold the guarantee for making sun-powered chargers that can be effortlessly kept on most surfaces, including adaptable and finished ones. These materials would likewise be lightweight, modest in delivery, and as effective as the present driving photovoltaic materials, which are essentially silicon. They’re the subject of expanding examination and speculation, yet organizations hoping to scale their true capacity in all actuality do need to address a few extra obstacles before perovskite-based solar power cells can be financially cutthroat.
The term perovskite alludes not to a particular material, similar to silicon or cadmium telluride, other driving competitors in the photovoltaic domain, but to an entire group of mixtures. The perovskite group of sun oriented materials is named for its primary similitude to a mineral called perovskite, which was found in 1839 and named after Russian mineralogist L.A. Perovski.
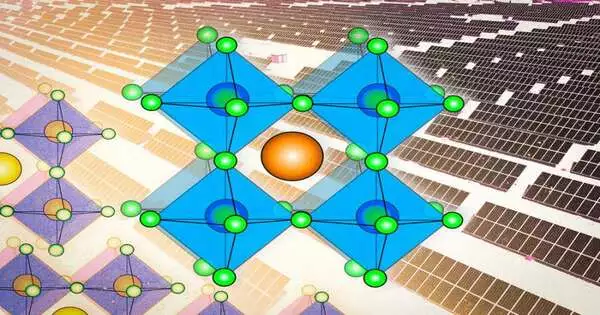
The first mineral perovskite, which is calcium titanium oxide (CaTiO3), has a particular precious stone setup. It has a three-section structure, whose parts have come to be named A, B, and X, in which the grids of the various parts are joined. The group of perovskites comprises the numerous potential mixes of components or particles that can possess every one of the three parts and structure a design like that of the first perovskite itself. (A few specialists even bend the guidelines a little by naming other gem structures with comparative components “perovskites,” albeit this is disapproved of by crystallographers.)
“With some restrictions, you can mix and match atoms and molecules to create the structure. For instance, you will disrupt the structure if you attempt to fit a molecule that is too large inside it. The 3D crystal may eventually split into a 2D layered structure or lose all ordered structure as a result of your actions.”
Tonio Buonassisi, professor of mechanical engineering at MIT
“You can blend and match iotas and particles into the design, for certain cutoff points.” For example, assuming you attempt to stuff a particle that is too enormous into the design, you’ll contort it. At last, you could make the 3D precious stone separate into a 2D layered structure, or lose the requested structure completely, “says Tonio Buonassisi, teacher of mechanical design at MIT and head of the Photovoltaics Research Laboratory.” “Perovskites are exceptionally tunable, similar to a form your-own-experience sort of gem structure,” he says.
That design of intertwined cross sections is made up of particles or charged atoms, two of which are strongly charged (An and B) and the other (X) is negatively charged.The An and B particles are normally of very different sizes, with the A being bigger.
Inside the general classification of perovskites, there are various sorts, including metal oxide perovskites, which have found applications in catalysis and in energy capacity and transformation, like in power modules and metal-air batteries. In any case, a primary focal point of the exploration movement for over 10 years has been lead halide perovskites, as per Buonassisi says.
Inside that class, there is as yet an army of conceivable outcomes, and labs all over the planet are dashing through the monotonous work of attempting to track down the varieties that show the best presentation in effectiveness, cost, and sturdiness—which has so far been the most difficult of the three.
Many groups have also focused on varieties that eliminate the use of lead in order to avoid its natural effect. Nonetheless, Buonassisi notes, in any case, that “reliably over the long haul, the toxic gadgets keep on working on their presentation, and none of the different sytheses draw near regarding electronic execution.” Work progresses forward, investigating options, but for the time being, none can contend with the lead halide renditions.
One of the extraordinary benefits perovskites offer is their extraordinary resistance to deformities in the design, he says. Dissimilar to silicon, which requires very high immaculateness to work well in electronic gadgets, perovskites can work well even with various blemishes and debasements.
Looking for encouraging new competitor structures for perovskites is a bit like searching for a difficult to find little item. However, as of late, specialists have thought of an AI framework that can enormously smooth out this interaction. This new methodology could prompt a lot quicker improvement of new options, says Buonassisi, who was a co-creator of that exploration.
While perovskites continue to demonstrate incredible commitment, and a few organizations are currently preparing to begin some business creation, solidity remains the most significant challenge they face.Perovskites degrade much faster than silicon-based chargers, which can retain up to 90% of their power yield for up to 25 years.Incredible headway has been made—beginning examples endured a couple of hours, then, at that point, weeks or months; more current definitions have usable lifetimes of up to a couple of years, making them reasonable for certain applications where life span isn’t fundamental.
According to an exploration viewpoint, Buonassisi says, one benefit of perovskites is that they are moderately simple to make in the lab—the compound constituents gather promptly. But on the other hand, that is their disadvantage: “The material goes together effectively at room temperature,” he says, “yet it additionally falls to pieces effectively at room temperature.” What is easy to get is never really appreciated. “
To manage that issue, most specialists are focused on utilizing different sorts of defensive materials to typify the perovskite, safeguarding it from openness to air and dampness. However, others are concentrating on the specific systems that lead to that corruption in order to find details or medicines that are all the more intrinsically strong. A key finding is that an interaction called autocatalysis is generally to blame for the breakdown.
In autocatalysis, when one piece of the material begins to corrupt, its response items go about as impetuses to begin debasing the adjoining portions of the construction, and an out of control response starts. A comparative issue existed in the early exploration of a few other electronic materials, like natural light-emanating diodes (OLEDs), and was ultimately tackled by adding extra sanitization moves toward the unrefined components, so a comparable arrangement might be tracked down on account of perovskites, Buonassisi recommends.
As of late, Buonassisi and his co-specialists finished a review showing that once perovskites arrive at a usable lifetime of essentially 10 years, because of their much lower starting expense, that would be adequate to make them monetarily suitable as a substitute for silicon in huge, utility-scale sun-oriented ranches.
Generally speaking, progress in the improvement of perovskites has been great and empowering, he says. He says that with only a couple of long stretches of work, it has proactively accomplished efficiencies tantamount to levels that cadmium telluride (CdTe), “which has been around a while, is as yet attempting to accomplish,” he says. “The simplicity with which these better exhibitions are arrived at in this new material is practically stunning.” He says that comparing how much exploration time is spent to accomplish a 1 percent improvement in productivity, he says, the advancement on perovskites has been somewhere close to 100 and multiple times quicker than that on CdTe. “That is one reason it’s so invigorating,” he says.