Manageable compound applications should have the option to utilize environmentally friendly power sources, sustainable natural substances, and earth-plentiful components. Nonetheless, to date, numerous strategies have been conceivable with the utilization of costly valuable metals or uncommon earth metals, the extraction of which can have serious natural effects. A group of scientists, including Professor Katja Heinze and Professor Christoph Kerzig of Johannes Gutenberg University Mainz (JGU) as well as Dr. Ute Resch-Genger of the German Bundesanstalt für Materialforschung und -prüfung (BAM), has now accomplished a leap forward in the utilization of chromium, a plentiful base metal which Heinze’s group has been examining for quite a while.
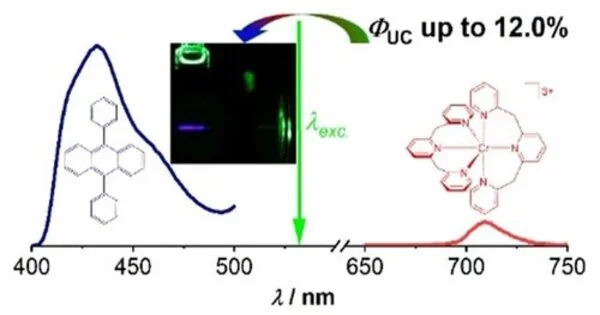
The new discoveries demonstrate the way that chromium compounds, also called atomic rubies, can substitute for costly valuable metals in photon upconversion. Photon upconversion (UC) is a cycle where the successful retention of two photons of lower energy prompts the outflow of one photon of higher energy. On a basic level, this higher energy photon can be used to increase the utilization of low-energy daylight in solar-powered cells or photochemical reactions, both of which require UV light for enactment. The utilization of atomic rubies can subsequently assist in diminishing the effect of ecologically harming cycles like the mining of valuable metals or intriguing earth components and can extend photochemistry to additional reasonable cycles.
Chromium compounds as a promising other option
Generally, photochemical and photophysical applications, for example, glowing natural light-transmitting diodes, color sharpened sunlight-based cells, or light-determined compound responses, utilize valuable metals like gold, platinum, ruthenium, iridium, or interesting earth metals. However, valuable metals are costly because they are scarce, and uncommon earth components are only mined in a few countries, specifically China. Moreover, their extraction frequently includes impressive utilization of water, energy, and synthetic compounds. For example, in gold mining, exceptionally poisonous substances, for example, cyanide or mercury, are utilized.
“In the process, we discovered a novel mechanism and gained a thorough understanding of the new chromium compounds’ exceptional efficiency,”
Professor Christoph Kerzig
Then again, the assets of the metal chromium, which gets its name from the old Greek word for variety, are multiple times more abundant in the Earth’s outside layer than those of platinum and multiple times more prominent than those of iridium, implying that it is accessible in adequate amounts. Sadly, the photophysical properties of plentiful metals like chromium or iron are simply not adequate to be helpful in mechanical applications, particularly with regards to the lifetimes and energies of their electronically energized states,” made sense by Professor Katja Heinze of JGU’s Department of Chemistry. A huge headway in such a manner has been made exclusively over the most recent couple of years, with Heinze’s group being one of the fundamental benefactors. They were additionally engaged with the development of supposed atomic rubies. These are solvent sub-atomic mixtures that have outstandingly great energized state attributes. Atomic rubies have previously been utilized as sub-atomic optical thermometers and strain sensors.
The new large scope laser gadget allows for direct perception of energy moving processes.
The group of researchers from Mainz and Berlin has now accomplished one more forward leap. “All the while, we noticed an original system and comprehended the high proficiency of the new chromium intensifier exhaustively,” said Professor Christoph Kerzig. The researchers discovered the unusual energy move pathway by utilizing a laser arrangement that was recently introduced in the Kerzig bunch. This supposed laser streak photolysis procedure permitted them to recognize all the intermediates that are significant for the upconversion systems. Moreover, quantitative laser tests laid out the shortfall of intrinsic energy misfortune channels and side responses, which lays the justification for the effective utilization of this underexplored method for moving and converting sun-oriented energy with chromium compounds.
Subsequently, researchers might have the option to foster new light-determined responses involving the normal metal chromium in the future as opposed to utilizing the uncommon, more expensive ruthenium and iridium compounds, which today are as yet the most commonly utilized. “Along with our accomplices at BAM in Berlin and different colleges, we will keep on pushing on with our endeavors to foster a more supportable photochemistry,” said Professor Katja Heinze.
The gathering’s outcomes have been published in Angewandte Chemie.