Zinc batteries may have a lower fire risk than their market-leading but fire-prone lithium-particle cousins, but experts believe the technology requires a significant boost in execution on multiple fronts if it is to live up to its promise.
A survey paper depicting the condition of zinc battery improvement was distributed to meet research needs. Survey articles—a profound plunge into the writing regarding a matter—are a critical piece of the cutting-edge logical strategy that assists scientists with explaining the ongoing grasping across a field and, significantly, recognizing holes in information.
The audit paper was distributed by Nano Exploration Energy.
As the clean transition away from petroleum derivatives has begun to take hold, the world has seen a significant increase in the sending of lithium-particle batteries, both for electric vehicles and for energy capacity to help with the sponsorship up factor for sustainable wellsprings of power, for example, wind and sunlight-based.
This development in the use of lithium-particle batteries has additionally expanded openness to a portion of the disadvantages of this innovation, not least the potential for this sort of battery to overheat and burst into flames, some of the time with destructive outcomes. Titles proliferate on media source articles itemizing loft fires and their casualties, with certain purviews considering prohibitions on e-bikes and e-bikes in structures. This is before the general execution of matrix-based energy capacity.
“Zinc has a long battery history, having been utilized as an anode material as early as 1799. One-third of the entire battery market is already accounted for by zinc-based battery technology.”
Dapeng Liu, a battery researcher with the Key Laboratory of Bio-inspired Smart Interfacial Science and Technology at Beihang University.
On top of their fire danger, lithium-particle batteries remain moderately costly (going from $400 to $800 per kilowatt per hour) and have a low unambiguous energy thickness (measure of energy that can be stored per kilogram). The low, unambiguous energy thickness of lithium-particle batteries specifically is the reason it remains so difficult to zap long-stretch delivery and flight: the epic size and weight of batteries adequate to take a boat or plane across a sea is the essential explanation that these two areas remain obstinately impervious to decarbonization.
Paradoxically, fluid batteries, specifically zinc/nickel (Zn/Ni), zinc/manganese (Zn/Mn), iron/nickel (Fe/Ni), and iron/cobalt (Fe/Co) ones, are lower cost and have high ionic conductivity. Because of the electrochemical responses engaged with their electrolytes (the medium that allows particle transport between the negative anode where current enters the battery and its positive partner, the cathode, where current exits the battery), zinc batteries specifically eliminate any risk of fire.
“Zinc has a strong history, having been used as an anode material as early as 1799,” said Dapeng Liu, a battery specialist at Beihang College’s Critical Research Facility of Bio-motivated Shrewd Interfacial Science and Innovation.”Zinc-based battery innovation, as of now, represents 33% of the world battery market.”
Zinc air batteries (ZABs)—whose design is somewhat open to utilize oxygen straightforwardly from surrounding air as its cathode reactant—hhave been sent for utility-scale energy capacity. ZABs have nearly multiple times the energy density of lithium-ion batteries and are manufactured at a significantly lower cost than the market leader.
Yet, assuming that there are these other battery choices, for what reason is the lithium-particle assortment so famous? According to the response, the last option outperformed almost every other challenger in terms of battery limit, or the number of hours for which the battery can deliver its full, planned (“nameplate”) volume of current.Worse, battery re-energizing lifetime is around 150 cycles under flow-down to earth conditions, and zinc-based batteries suffer from low coulombic productivity (how much electric charge is put into a battery compared to the total charge extricated from it).
If zinc batteries, with their superior fire safety record, triumph over lithium-ion batteries, critical advancements in battery limit and future should be made, fulfilling the requirement for the survey paper.
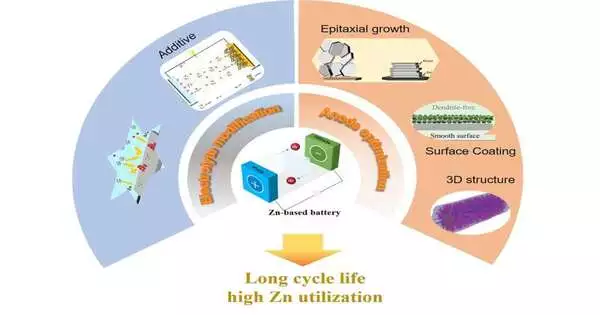
The survey creators presumed that the chief purposes behind the low cycle life of zinc batteries are threefold: the uncontrolled anode-based development of spiky shards of zinc called dendrites that can impede batteries; undesirable side-effects of cycling, for example, zinc oxide whose compound responses delivering them can’t be switched; and a consistent utilization of electrolytes.
The greater part of the examination pointed toward working on the presentation of zinc batteries in this manner, which centers basically around the change of the zinc anode and cathode material plan and on further developing electrolytes.
The commentators inferred that momentum research highlights a range of approaches that have made some progress.
Concerning the anode, the commentators viewed that “development control,” “connection point change,” and “planning Zn cathode structures” were probably going to accomplish enhancements, like stacking the zinc onto a permeable 3D conductive skeleton, adjusting the connection point between the anode and the electrolyte, alloying the zinc with different metals, and adding added substances, which have demonstrated to be the best strategies at further developing zinc anode execution.
Concerning the electrolyte, because it is the exhaustion or weakening of the fluid electrolyte that is responsible for the last disappointment of most zinc batteries, advancement of this component may be more critical, in general, to superior business suitability than any other viewpoint.Here, the exploration is pointing towards new, elite execution and stable electrolytes.
For fluid electrolytes, added substances and economical surfactants (substances that lessen surface strain) ought to work on their electrochemical exhibitions. The ionic fluids have inborn wellbeing, steadiness, and a great many electrochemical possibilities, which can fill in for conventional watery electrolytes. Zinc batteries that use strong electrolytes rather than fluid ones have excellent warm dependability and high actual adaptability.
The analysts reasoned that the exhibition of zinc-based batteries has worked exceptionally well recently because of a large portion of the research covered in their survey. Regardless of this advancement, they find that battery-powered zinc batteries stay mismatched for the overwhelming majority of business applications. They trust that their survey offers accommodating signs to the field showing where the principal research holes are untrue and which roads at present show the most commitment.
More information: Huaiyun Ge et al, Recent advances and perspectives for Zn-based batteries: Zn anode and electrolyte, Nano Research Energy (2022). DOI: 10.26599/NRE.2023.9120039. www.sciopen.com/article/10.26599/NRE.2023.9120039