Logical examination requires tolerance. The prizes are not generally quick, and the innovation required doesn’t necessarily exist in every case. Michael A. Trakselis, Ph.D., teacher and overseer of graduate undertakings for the Branch of Science and Organic Chemistry at Baylor College, figures this out.
For quite a long time, Trakselis has been keen on the MCM8/9 protein. Part of the minichromosomal support protein (MCM) family, MCM8/9 is straightforwardly connected to ovarian inadequacy, fruitlessness, and malignant growths, including ovarian, testicular, and colon tumors, yet little is known about the way this functions and its association with illness. There was no comprehension of the connection between the construction and capability of the compound.
The examination—movement, substrate inclination, and design of the HsMCM8/9 helicase—was distributed in the diary Nucleic Acids Exploration. With this new data, specialists in the Trakselis Lab and somewhere else have a guide to all the more likely comprehend the capability of this catalyst complex and how changes might be associated with sickness.
“Transformations in MCM8 or MCM9 can cause fruitlessness and malignant growth; yet, we don’t have the foggiest idea why we get specific illnesses when there are changes in this protein,” Trakselis said. “Having the construction could assist us with sorting out a portion of that.”
Before that could occur, Trakselis needed to overcome two boundaries: innovation and a steady protein arrangement expected to direct the examination.
Up to this point, the innovation expected to grasp MCM8/9 was not promptly accessible. The single-molecule cryo-transmission electron magnifying instrument (cryo-EM) is an exceptionally delicate and costly magnifying lens that has been accessible for use for around eight years.
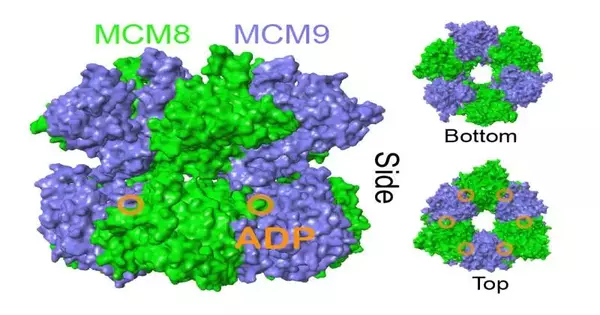
PC produced a model of the MCM8/9 compound. Credit: Baylor College
“It’s a monstrous instrument, yet it attempts to picture truly little things,” Trakselis said.
The cryo-EM imagines and tackles what the construction resembles by shooting electrons into the material. “In view of how the bars are reflected or refracted, you can begin to fabricate a construction.”
On this examination, teaming up with specialists from Rice College and the College of Texas Wellbeing Science Framework in Houston, Dr. Trakselis was capable of accessing a cryo-EM.
The following boundary was a steady and unadulterated protein arrangement. An unclean or shaky example would prevent the cryo-EM from uncovering any exact primary data on the MCM8/9 complex. Trakselis and his group attempted a few ways to deal with communicating and sanitizing different developments of MCM8 and 9, but they were of low virtue and solvency. After much experimentation, the group eventually attempted bug cells for articulation of this human protein, which at long last brought about stable, unadulterated protein that permitted cryo-EM imaging of the MCM8/9 complex.
With the hindrances eliminated, Trakselis and his group had the option to disentangle the construction of MCM8/9 bound to its substrate item, ADP.
For Trakselis, this is just the start. There are more inquiries to be responded to.
“We depicted the fundamental construction capability relationship, yet we don’t have the foggiest idea what it does at the cell level and in what setting,” Trakselis said.
More information: David R McKinzey et al, Activity, substrate preference and structure of the HsMCM8/9 helicase, Nucleic Acids Research (2023). DOI: 10.1093/nar/gkad508