Creating a comprehensive insulin signaling map that includes the interaction of genes and diet is a difficult task that necessitates a thorough understanding of molecular biology, genetics, and nutritional science. A map like this would aid academics and physicians in better understanding the complex mechanisms underlying insulin signaling and its modulation in response to genetic and dietary effects. Researchers have created a comprehensive picture of insulin signaling in mice, claiming that it is affected by the interplay of heredity and nutrition.
The editors regard the research, which was published today as a Reviewed Preprint in eLife, as a foundational study of significant importance. According to the authors, they present persuasive evidence that sheds light on the interplay between genetic characteristics and environmental factors in determining insulin signaling in skeletal muscle – a critical regulator of metabolism. The work also gives a one-of-a-kind tool for monitoring the range of phosphorylation in insulin reactions, and it is expected to inspire further research into metabolic illness and diabetes.
“Insulin resistance – the failure of insulin to promote glucose uptake in its target tissues – is triggered by genetic and environmental factors such as family history and high-calorie diets,” says lead author Julian van Gerwen, an undergraduate at the University of Sydney’s School of Life and Environmental Sciences. “Although insulin resistance is a major precursor of metabolic disease, including type 2 diabetes, its mechanistic basis remains unresolved.”
Insulin resistance – the failure of insulin to promote glucose uptake in its target tissues – is triggered by genetic and environmental factors such as family history and high-calorie diets. Although insulin resistance is a major precursor of metabolic disease, including type 2 diabetes, its mechanistic basis remains unresolved.
Julian van Gerwen
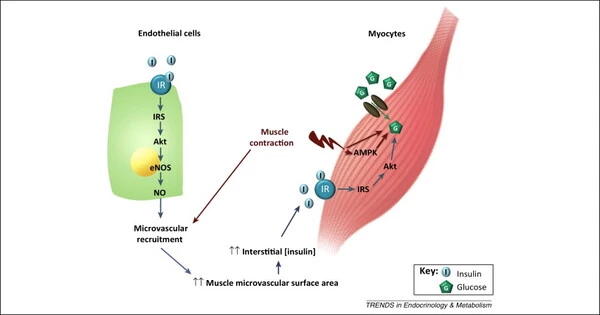
Insulin ordinarily instructs the body via a complex and dynamic signaling system to absorb glucose (sugar) from the bloodstream. These signals are made possible by a process known as phosphorylation, which involves the attachment of a phosphate group to a protein at a very particular location (known as a phosphosite).
Insulin signaling is thought to control thousands of phosphosites, however many remain unidentified. Furthermore, while it is generally understood that individual persons differ substantially in their physiological response to insulin, it is unclear how genetics or nutrition influence the phosphorylation status of cellular proteins, also known as the phosphoproteome.
Van Gerwen and colleagues investigated this by studying mice with well-characterized but varied genetic backgrounds in order to determine the precise impacts of genetics and nutrition on insulin signaling. They fed five strains of mice a normal or high-fat, high-sugar diet and collected skeletal muscle samples, which is the site of maximum insulin-triggered glucose uptake after eating. Then they measured phosphorylation of the thousands of proteins present in each muscle sample using mass spectrometry. Their analysis recovered many well-known insulin-regulated phosphosites, and many more novel sites that had not previously been associated with insulin signalling.
To investigate the impact of genetic and environmental variation, the researchers created an algorithm to determine which changes may be linked to genetics, food, or a mix of the two. When fed a normal diet, the strain of mice affected nearly half of all insulin-regulated phosphosites, either having a higher or lower response to insulin. Overall, each genetic background exhibited a distinct fingerprint of insulin signaling.
In contrast, while there were modifications in insulin signaling driven by diet, the great majority of these were shaped by the mice’s genetic background. Many phosphosites even shifted in the opposite way across multiple strains, demonstrating that the molecular effects of a high-fat diet are substantially influenced by genetics.
To explore whether these changes in phosphorylation amounted to an altered insulin response in the mice, the team also measured glucose uptake in the same muscles used for the phosphoproteome analysis. By linking all insulin-regulated phosphosites with the level of glucose uptake, the researchers narrowed down on a set of key phosphosites likely to control the insulin response. Inspired by one of these phosphosites, the team found that modulating a specific protein could reverse insulin resistance in a cell-based model.
The authors point out that the existing model of the insulin signaling system cannot predict the genetic and dietary alterations in phosphorylation. This demonstrates that our understanding of this route is far from complete, and the next step is to examine underlying biomolecular mechanisms that potentially link common changes. They also believe that integrating female mice and a broader range of genetic origins will boost their research.
“The protein phosphorylation landscape is vast and intricate, akin to a night sky filled with stars,” explains senior author David James, a professor at the University of Sydney’s School of Life and Environmental Sciences and Faculty of Medicine and Health. “Many teams have sought to organise these stars into constellations and chart those that collapse in disease. However, most have only used cell lines and lab animals of limited genetic backgrounds.
In this study, we found a total reorganization of the night sky, highlighted by the fading of recognizable constellations and the creation of wholly new galaxies, when we evaluated genetic and environmental variation, as occurs in the human population. To really understand how diseases manifest from signaling abnormalities, we must adjust to this greater complexity. Our findings pave the way for further research into the complexities of insulin resistance and diabetes.”