Bacterial infectious diseases are still a major contributor to global disease, and with anti-toxin opposition on the rise, there is an urgent need for novel anti-microbe therapy systems.One of the most devastating bacterial diseases is cholera, brought about by the microbes Vibrio cholerae, which has been in its seventh continuous pandemic starting around 1961. An examination group driven by Osaka College in Japan has revealed insight into a particular protein connection that can possibly be a clever objective in cholera treatment.
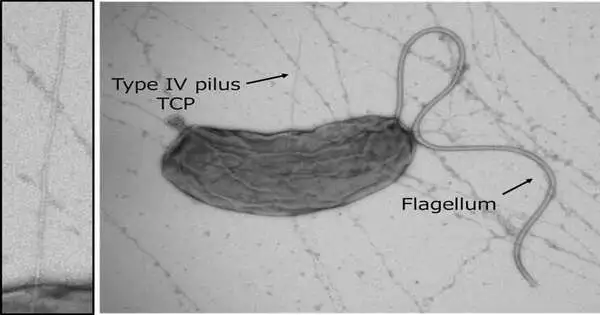
Cholera is described by extreme diarrhea that can be deadly in the space of hours, to say the least. Perhaps one of the most important steps in the V. cholerae disease cycle is for the bacterium to colonize the human digestive tract by emitting a colonization factor called TcpF, but the specific system behind this emission has remained obscure.In the present, in a review that will be published soon in Science Advances, scientists utilized X-beam crystallography, physicochemical examinations, and primary display to uncover precisely how V. cholerae secretes TcpF.
“It was known that the Poison coregulated pilus (TCP), a sort of 4 pilus framework, assumed a vital part in TcpF emission, yet the specific connection between the two was hazy,” says Hiroya Oki, lead creator of the review. Pili are fiber like designs on the outer layer of bacterial cells that can have a huge number of capabilities. The V. cholerae TCP is made basically out of various TcpA subunits, with an underlying minor subunit including a TcpB trimer joining to the “top” of the pilus to work with its gathering. The gathering concentrated on the connection of TcpF with TcpA and B and made models in light of the outcomes.
“We saw that TcpF trimerized into a bloom like unit to tie to the TcpB trimer toward the finish of the pilus,” makes sense for Shota Nakamura, senior creator of the review. “Critically, we recognized separate saved spaces that are crucial for the restricting of TcpF to TcpB and TcpF trimerization, the two of which are expected for V. cholerae colonization.”
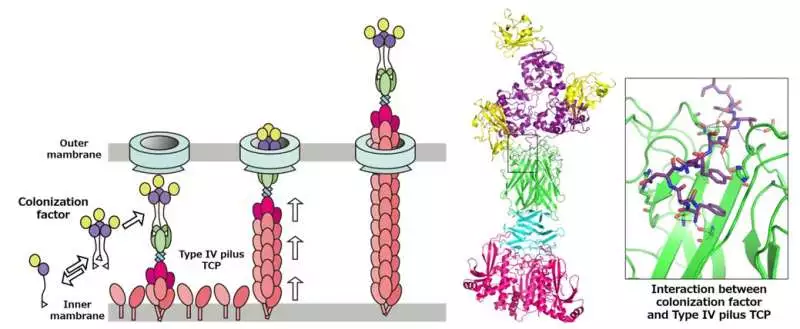
Left: Model of the Kind IV pilus framework moving the solvent colonization factor. Right: Gem design of TCP in the complex with the colonization factor. Credit: 2022 Oki et al., Science Advances
While considering their discoveries in context with other distributed works, the group guessed a model of emission where TCP does TcpB-bound TcpF of the phone, after which TcpF separates from the pilus and moves openly in the human digestive tract, starting the beginning phases of V. cholerae colonization. TCP then withdraws once more into the bacterial cell to rehash the cycle.
Given the developing protection from anti-toxins, discoveries like these that explain the atomic subtleties of disease can be profoundly important for planning new antibacterial medications. The improvement of an enemy of glue specialist that specifically hinders the connection between the TCPF colonization factor and the TCP emission framework could provide a clever treatment system to battle cholera.
The article, “Primary reason for the poison coregulated pilus-subordinate emission of Vibrio cholera colonization factor,” will be distributed in Science Advances.
More information: Hiroya Oki et al, Structural basis for the toxin-coregulated pilus–dependent secretion of Vibrio cholerae colonization factor, Science Advances (2022). DOI: 10.1126/sciadv.abo3013. www.science.org/doi/10.1126/sciadv.abo3013
Journal information: Science Advances